A kind of s-carboxymethyl-l-cysteine enteric-coated pellet capsule
A technology of cystine injection and cysteine, which is applied in the direction of microcapsules, capsule delivery, drug combination, etc., can solve the problems of unresolved drug side effects, validity period of only 24 months, poor stability, etc., to improve bioavailability High degree, easy to store, improve the effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
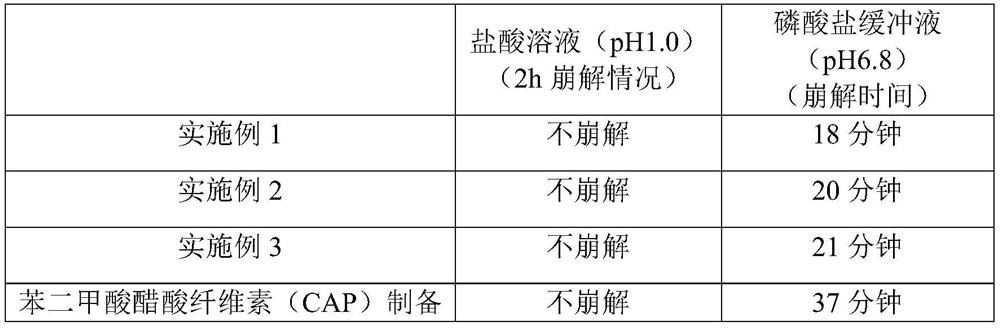
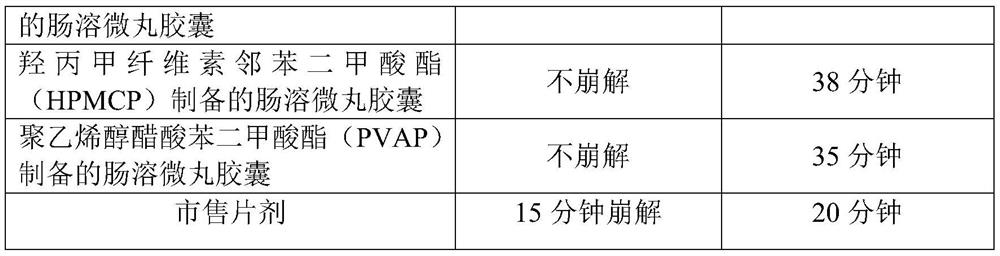
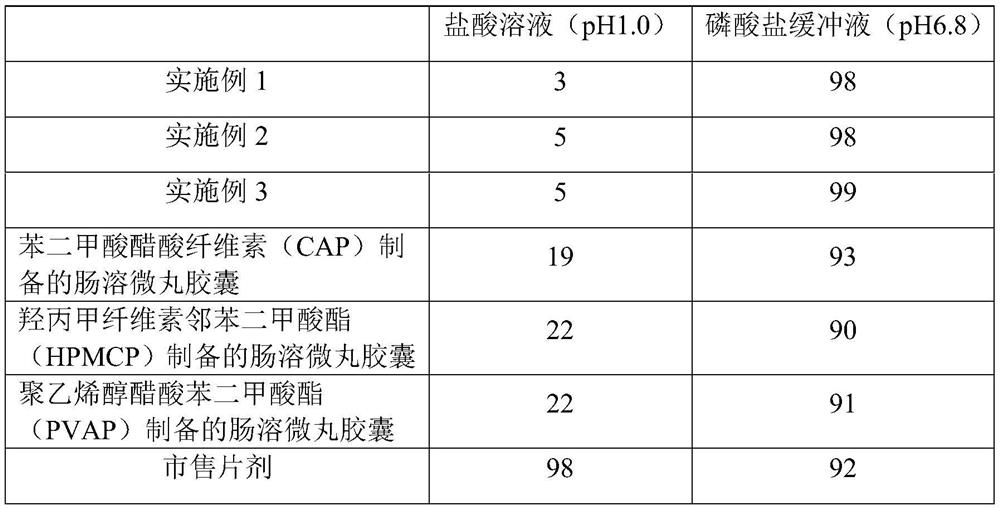
Examples
Embodiment 1
[0020] Ball core
[0021] S-carboxymethyl-L-cysteine 250mg;
[0022] Carbomer 100mg;
[0023] Hypromellose 80mg.
[0024] Isolation layer
[0026] Hypromellose 5mg;
[0027] Polyethylene glycol 2mg.
[0028] Enteric layer
[0029] Waterborne acrylic enteric coating system 93A 30mg;
[0030] Simethicone 0.3mg;
[0031] PEG8000 3mg.
[0032] Preparation method:
[0033] (1) Pill core containing medicine: take the hypromellose of prescription quantity, add water and dissolve to be binder; Take the S-carboxymethyl-L-cysteine and carbomer of prescription quantity, mix evenly, pass 100-mesh sieve, soft material made of hypromellose solution as binder. Start the extrusion and spheronization equipment, select the aperture of the sieve plate to be 0.8mm, and the rotation speed to be 4000 rpm, and extrude and spheronize to obtain pellets; dry and pass through a 20-mesh sieve to obtain the pellet core.
[0034] (2) Isolation layer: Weigh the pres...
Embodiment 2
[0038] Ball core
[0039] S-carboxymethyl-L-cysteine 250mg;
[0040] Carbomer 50mg;
[0041] Hypromellose 30mg.
[0042] Isolation layer
[0044] Hypromellose 2mg;
[0045] Polyethylene glycol 1mg.
[0046] Enteric layer
[0047] Waterborne acrylic enteric coating system 93A 20mg;
[0048] Simethicone 0.2mg;
[0049] Triethyl Citrate 2mg.
[0050] Preparation method:
[0051] (1) Pill core containing medicine: take the hypromellose of prescription quantity, add water and dissolve to be binder; Take the S-carboxymethyl-L-cysteine and carbomer of prescription quantity, mix evenly, pass 100-mesh sieve, soft material made of hypromellose solution as binder. Start the extrusion and spheronization equipment, select the aperture of the sieve plate to be 0.8mm, and the rotation speed to be 4000 rpm, and extrude and spheronize to obtain pellets; dry and pass through a 20-mesh sieve to obtain the pellet core.
[0052] (2) Isolation layer: Weigh th...
Embodiment 3
[0056] Ball core
[0057] S-carboxymethyl-L-cysteine 250mg;
[0058] Carbomer 10mg;
[0059] Hypromellose 10mg.
[0060] Isolation layer
[0062] Hypromellose 2mg;
[0063] Polyethylene glycol 2mg.
[0064] Enteric layer
[0065] Water-based acrylic enteric coating system 93A 20mg;
[0066] Simethicone 0.2mg;
[0067] Glyceryl Triacetate 3mg.
[0068] Preparation method:
[0069] (1) Pill core containing medicine: take the hypromellose of prescription quantity, add water and dissolve to be binder; Take the S-carboxymethyl-L-cysteine and carbomer of prescription quantity, mix evenly, pass 100-mesh sieve, soft material made of hypromellose solution as binder. Start the extrusion and spheronization equipment, select the aperture of the sieve plate to be 0.8mm, and the rotation speed to be 4000 rpm, and extrude and spheronize to obtain pellets; dry and pass through a 20-mesh sieve to obtain the pellet core.
[0070] (2) Isolation layer: We...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com