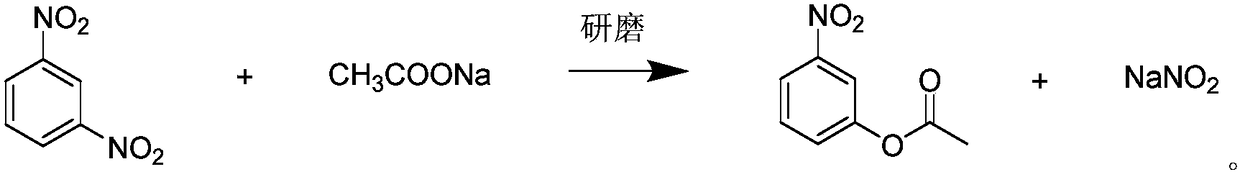
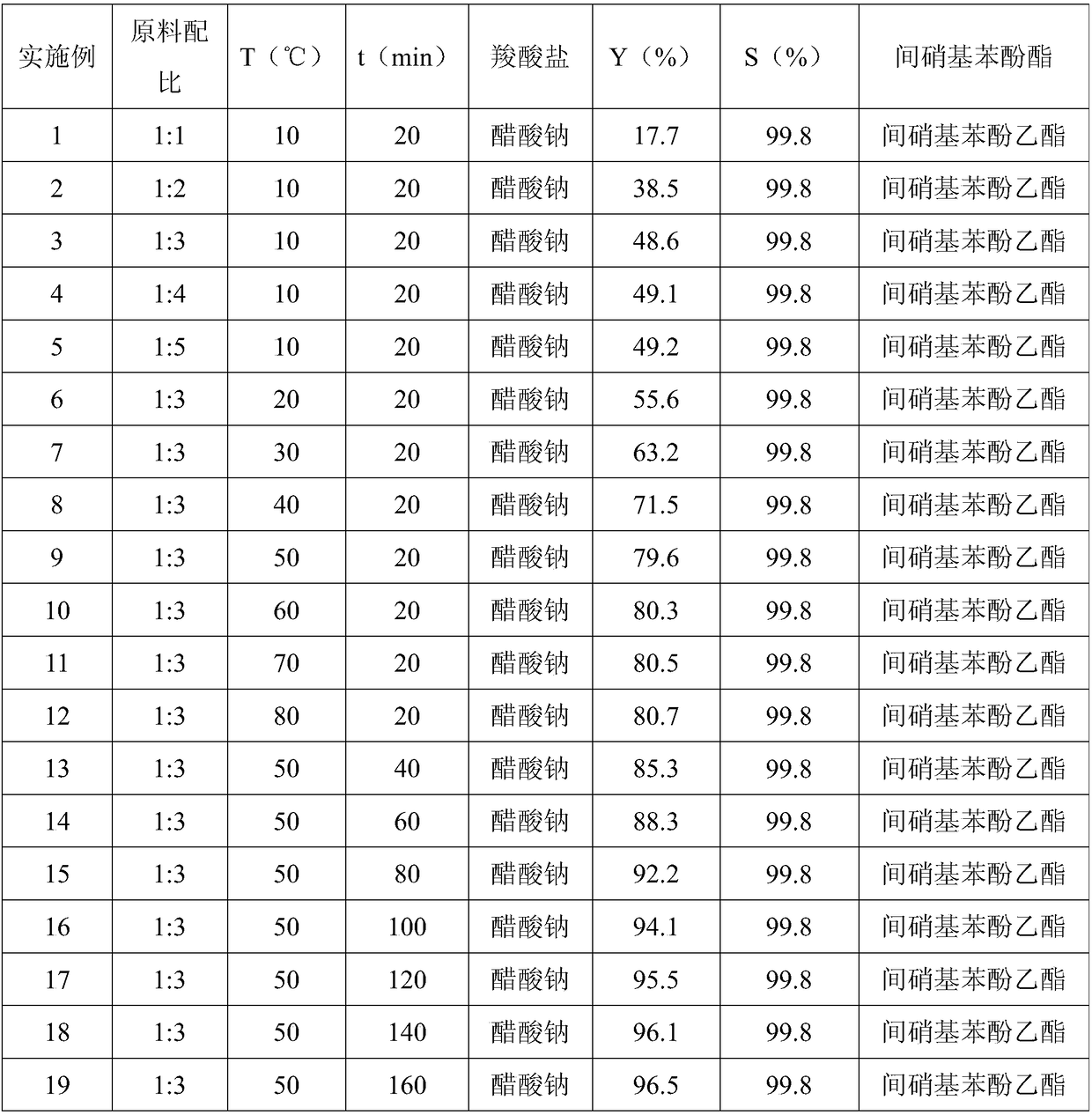
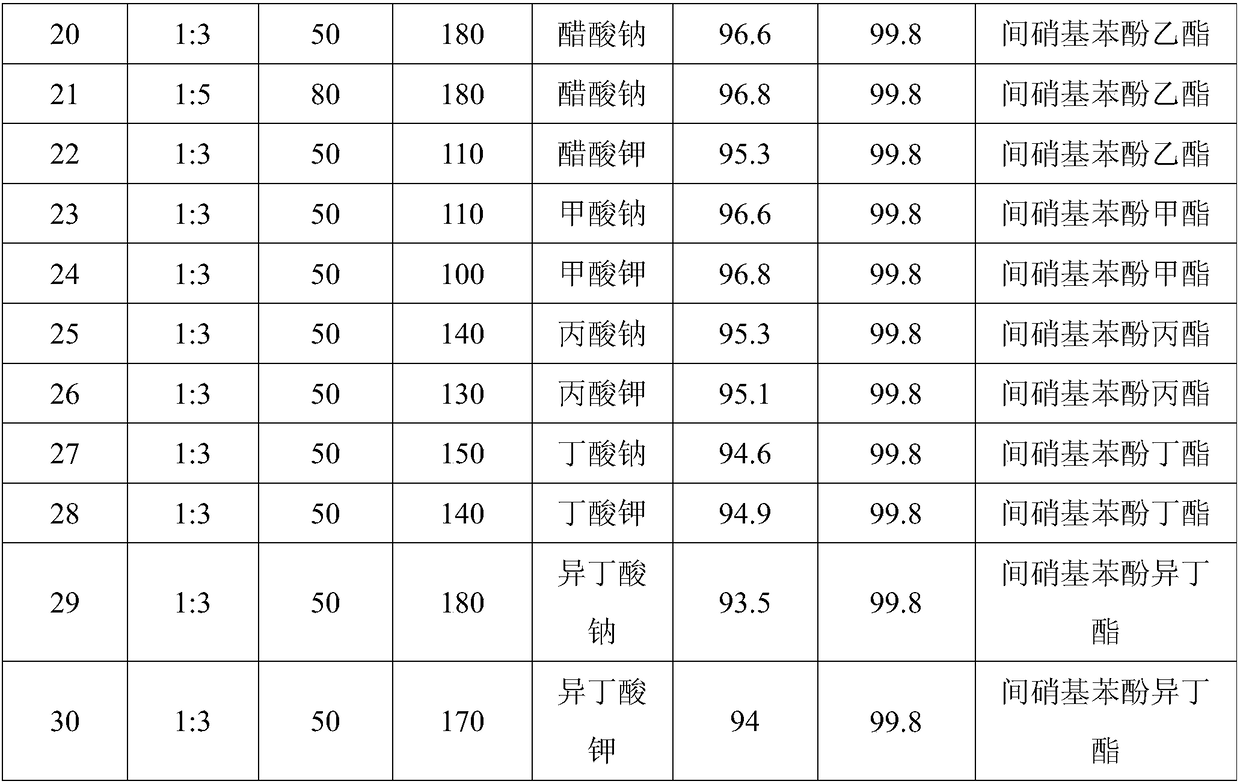
Method for preparing m-nitrophenol ester by solid-phase reaction system
A technology of nitrophenol ester and solid phase reaction, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of complex preparation methods, high toxicity of acetonitrile, high price, etc., and achieve simple post-processing, The effect of less toxic side effects and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1, a kind of preparation method of ethyl m-nitrophenolate, take m-dinitrobenzene and sodium acetate as raw material, carry out the following steps successively:
[0022] 1) Take 16.8g (0.1mol) of m-dinitrobenzene and 8.2g (0.1mol) of sodium acetate, put them in a 50mL stainless steel screw cap grinding jar, seal it and fix it on the Mixer Mill 400 mixing mill , set the vibration frequency to 30 Hz, and grind at 10°C for 20 min.
[0023] 2), wash with 150mL of anhydrous methanol after the reaction, filter, repeat 3 times, combine the filtrate, reduce pressure (vacuum degree is 40mmHg) after distilling methanol in the filtrate, column chromatography separates the product;
[0024] The column chromatography is as follows: 20-50 g of 200-300-mesh column chromatography silica gel is installed in the glass chromatography column, and all the filtrate after distilling methanol is loaded, and ethyl acetate:petroleum ether=1:2 The mixed solution of (volume ratio) is a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com