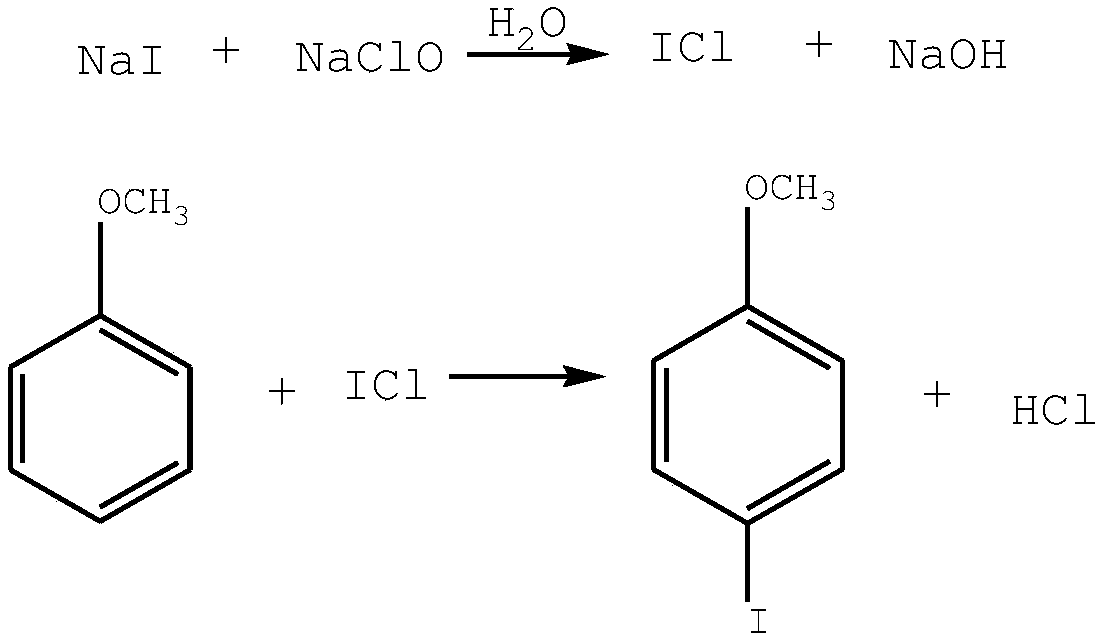
Synthetic method of 4-iodoanisole
A technology of iodoanisole and synthesis method, which is applied in the field of synthesis of 4-iodoanisole, can solve problems such as instability of iodine chloride and complex synthesis route, and achieve safe and controllable reaction, simple and convenient post-treatment, and green reaction Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] A kind of synthetic method of 4-iodoanisole, comprises the following steps:
[0023] (1) prepare 2 moles of 20% NaI aqueous solution A and 2.1 moles of 8-10% sodium hypochlorite aqueous solution B for subsequent use;
[0024] (2) Add 900g of anisole into a 2L four-necked round-bottomed flask equipped with stirring, temperature display, and two constant pressure dropping funnels, and heat to 60-70°C under stirring; Add sodium iodide solution and sodium hypochlorite solution dropwise at the same time in a ratio of 1:1.05;
[0025] (3) After the dropwise addition, raise the temperature to 75-80°C and stir for 1 hour to obtain a light yellow reaction solution; cool to room temperature, stand to separate the phases; wash the organic phase with water, dry and filter to obtain the benzidine of 4-iodoanisole Ether solution, rectification, cooling, and solidification to obtain white massive crystals, namely 4-iodoanisole, the content of which is ≥98.5%, and the yield is 93.4%. ...
Embodiment 2
[0027] A kind of synthetic method of 4-iodoanisole, comprises the following steps:
[0028] (1) prepare 2 moles of 20% NaI aqueous solution A and 2 moles of 8-10% sodium hypochlorite aqueous solution B for subsequent use;
[0029] (2) Add 800g of anisole into a 2L four-necked round-bottomed flask equipped with stirring, temperature display, and two constant pressure dropping funnels, and heat to 60-70°C under stirring; Add sodium iodide solution and sodium hypochlorite solution dropwise at the same time in a ratio of 1:1;
[0030] (3) After the dropwise addition, raise the temperature to 75-80°C and stir for 1 hour to obtain a light yellow reaction solution; cool to room temperature, stand to separate the phases; wash the organic phase with water, dry and filter to obtain the benzidine of 4-iodoanisole The ether solution was rectified, cooled and solidified to obtain white blocky crystals, namely 4-iodoanisole, the content of which was ≥97%, and the yield was 91.2%.
Embodiment 3
[0032] A kind of synthetic method of 4-iodoanisole, comprises the following steps:
[0033] (1) prepare 2 moles of 20% NaI aqueous solution A and 2.2 moles of 8-10% sodium hypochlorite aqueous solution B for subsequent use;
[0034] (2) Add 770g of anisole into a 2L four-necked round-bottomed flask equipped with stirring, temperature display, and two constant pressure dropping funnels, and heat to 60-70°C under stirring; Add sodium iodide solution and sodium hypochlorite solution dropwise at the same time in a ratio of 1:1.1;
[0035] (3) After the dropwise addition, raise the temperature to 75-80°C and stir for 1 hour to obtain a light yellow reaction solution; cool to room temperature, stand to separate the phases; wash the organic phase with water, dry and filter to obtain the benzidine of 4-iodoanisole Ether solution, rectification, cooling, and solidification to obtain white massive crystals, that is, 4-iodoanisole, the content of which is ≥97%, and the yield is 90.6%. ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap