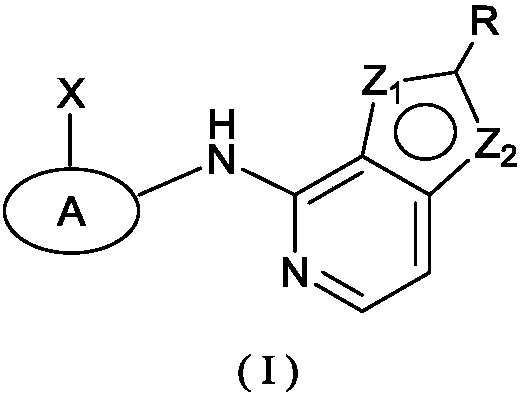
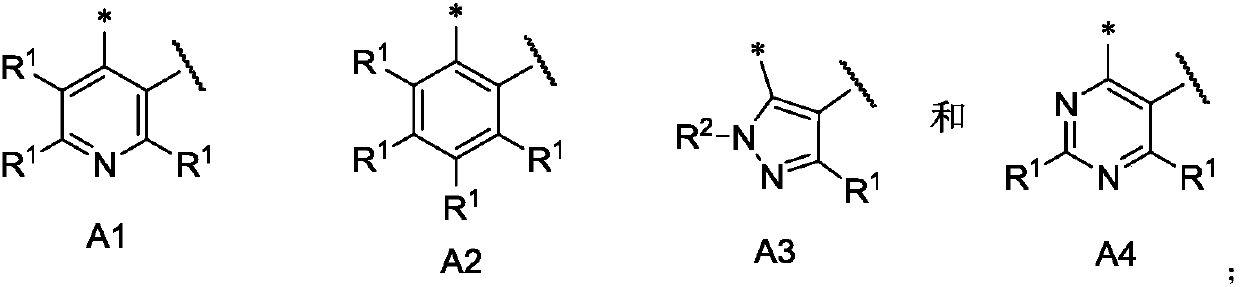
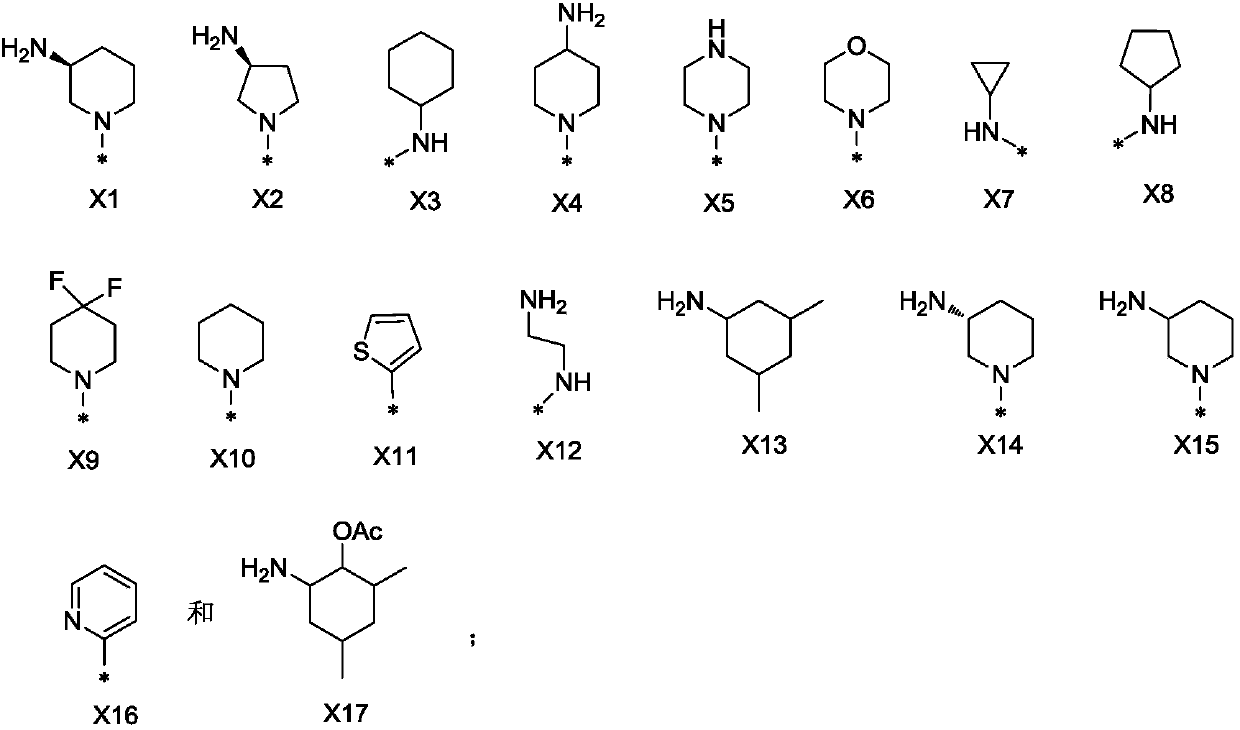
Kinase inhibitor, and preparation and application of kinase inhibitor
A solvate and compound technology, applied in the field of kinase inhibitors and its preparation and application, can solve the problems of long synthetic route and single optical isomer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0164] In the preparation of compounds of the present invention, it may be necessary to protect certain interfering functional groups (e.g., primary or secondary amines) of intermediates. The requirements for such protecting groups vary depending on the nature of the particular functional group and the conditions of the preparation method. Suitable amino-protecting groups include acetyl, trifluoroacetyl, tert-butoxycarbonyl (Boc), benzyloxycarbonyl (Cbz), 9-fluorenylmethyleneoxycarbonyl (Fmoc), and the like. Suitable hydroxy protecting groups include allyl, acetyl, silyl, benzyl, trityl, p-methoxybenzyl, and the like. Such protecting groups can be easily determined by those skilled in the art (for details, refer to Protective Groups in Organic Synthesis, John Wiley & Sons, New York, Third Edition, 1999).
[0165] Reaction Scheme 1 below provides a representative procedure for the preparation of compounds of formula (I). It should be understood that these reaction steps and c...
Embodiment 1
[0198] Example 1, (S)-N-(4-(3-aminopiperidin-1-yl)pyridin-3-yl)-2-(2,6-difluorophenyl)thiazolo [4,5-c]pyridin-4-amine
[0199] Preparation of (S)-1-(3-aminopyridin-4-yl)piperidin-3-yl-carbamic acid tert-butyl ester
[0200]
[0201] Step 1, preparation of (S)-1-(3-nitropyridin-4-yl)piperidin-3-yl-carbamic acid tert-butyl ester
[0202] 4-Chloro-3-nitropyridine (475.6mg, 3mmol), (S)-3-tert-butyloxycarbonylaminopiperidine (600.8mg, 3mmol) and DIPEA (387.7mg, 3.0mmol) were sequentially added to a 50mL circle Bottom flask, add 10mL of ethanol, and stir at room temperature for 5h. The reaction solution was concentrated under reduced pressure, the residue was dissolved in ethyl acetate, and the ethyl acetate phase (50 mL×3) was washed with water. The organic phase was dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The residue was separated and purified by silica gel column chromatography (petroleum ether: ethyl acetate = 3:1) to o...
Embodiment 2
[0223] Example 2, (S)-N-(4-(3-aminopiperidin-1-yl)pyridin-3-yl)-2-phenylthiazolo[4,5-c]pyridine- 4-amine
[0224] Preparation of 4-chloro-2-phenylthiazolo[4,5-c]pyridine
[0225]
[0226] Step 1, preparation of 3-amino-4-mercaptopyridine
[0227] Dissolve 4-chloro-3-nitro-pyridine (7.9g, 49.8mmol) in 50mL ethanol, slowly add 50mmol concentrated hydrochloric acid, stir at room temperature; then weigh NaHS·H 2O (13.69g, 184.8mmol) was added to the reaction mixture, stirred at room temperature for 40min; then sodium hydrosulfate (32.21g, 185.1mmol) was weighed and dissolved in water, and the aqueous sodium hydrosulfite solution was added to the reaction mixture, stirred at 80°C for 12h. The reaction solution was filtered, the filtrate was concentrated under reduced pressure, separated and purified by silica gel column chromatography (the impurities were first eluted with dichloromethane:methanol=5:1, and then the crude product was obtained by elution with dichlorometh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com