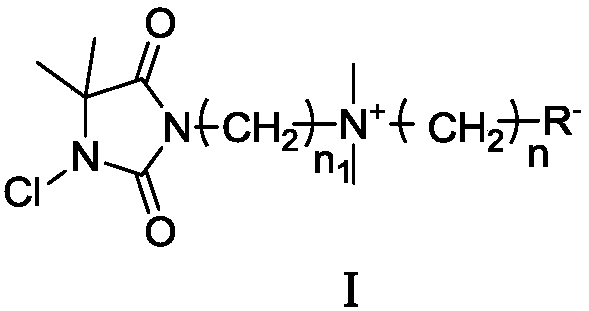
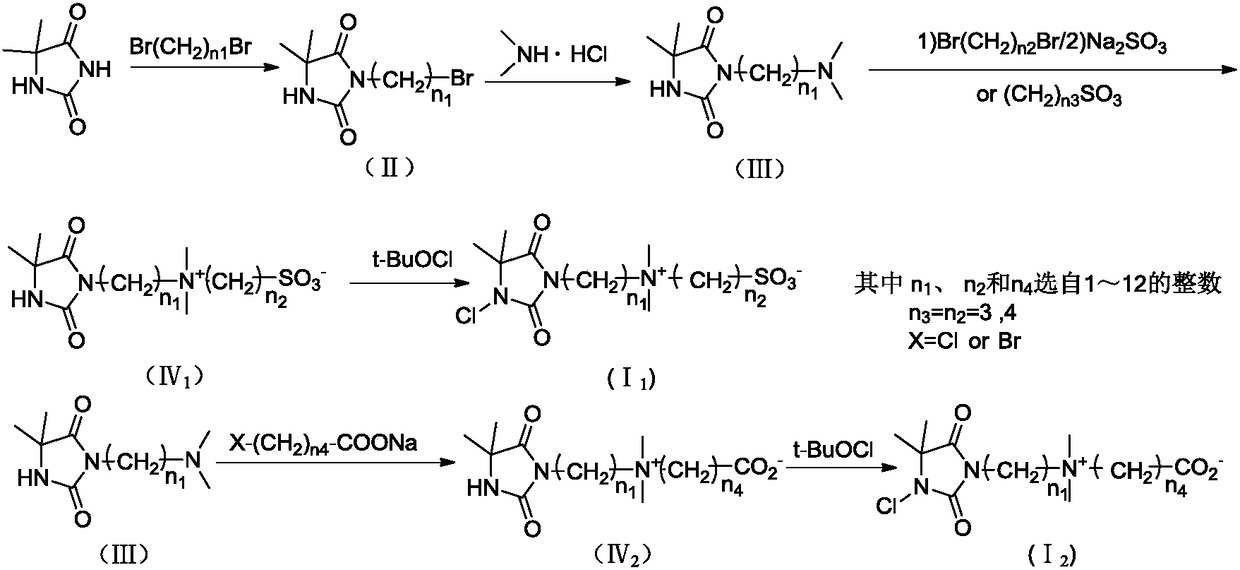
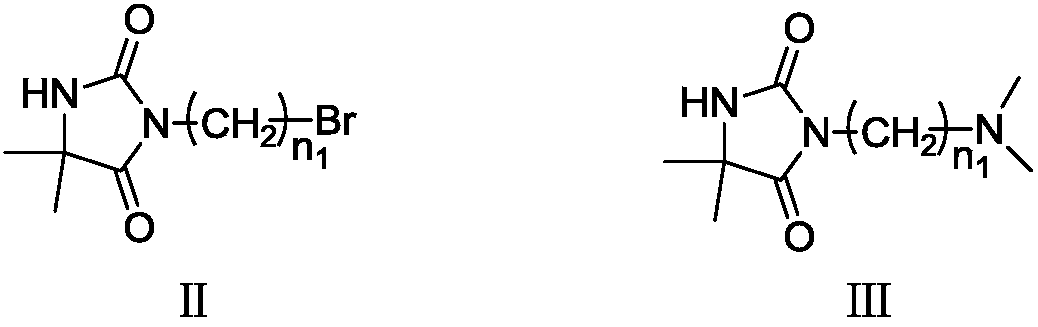Betaine type chloramine antibacterial agent and synthetic method thereof
A synthesis method and technology of betaine, which are applied in botany equipment and methods, biocides, disinfectants, etc., can solve problems such as poor dispersion, poor hydrophilicity of chloramine antibacterial agents, unfavorable sterilization process and sterilization effect, etc. To achieve the effect of improving water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Compound 1 (0.82g, 3.86mmol) was dissolved in 2mL of acetonitrile, then 1,2-dibromoethane (7.29g, 38.6mmol) was added, and heated to reflux for 24h. Concentrated under reduced pressure to remove the solvent, followed by column chromatography separation and purification, using methanol and dichloromethane (1 / 19, v / v) as eluents, collecting the organic phase containing the product to obtain compound 2 as a fluffy light yellow solid (1.06 g, 68.2%).
[0026] 1 H NMR (500HMz,D 2 O)δ3.76-3.73(m,2H),3.71-3.68(m,2H),3.52(t,J=6.8Hz,2H),3.37-3.34(m,2H),3.08(s,6H), 2.10-2.04(m,2H),1.35(s,6H); 13 CNMR (126MHz,D 2 O) δ180.6, 157.1, 63.9, 62.0, 59.2, 50.9, 35.3, 23.4, 21.3, 20.6. HRMS calcd.for C 12 h 23 N 3 o 2 Br[M-Br] + 320.0974,found: 320.0986.
Embodiment 2
[0028] Compound 2 (0.87g, 2.16mmol) was dissolved in 5mL of water, then sodium sulfite (0.43g, 3.40mmol) was added, and reacted at 90°C for 24h. After the reaction stopped, remove the water, add 12mL of methanol under heating conditions to dissolve, filter to remove insoluble salts, concentrate the filtrate under reduced pressure, carry out column separation and purification, and elute with methanol and dichloromethane (1 / 1, v / v) The organic phase containing the product was collected to give a fluffy solid (0.50 g, 72.6%). The solid was recrystallized, dissolved in about 10mL of methanol under heating, and about 70mL of acetone was added under stirring, a white floc was formed, and after standing, it was filtered to obtain compound 5 as a white solid (0.43g, 86%)
[0029] 1 H NMR (500HMz,D 2 O)δ3.64-3.60(m,2H),3.51(t,J=6.8Hz,2H),3.34-3.31(m,4H),3.07(s,6H),2.09-2.03(m,2H), 1.34(s,6H); 13 C NMR (126MHz,D 2 O) δ180.5, 157.0, 61.8, 59.3, 59.1, 50.9, 44.0, 35.2, 23.4, 21.3. HR...
Embodiment 3
[0031] Compound 5 (0.50g, 1.57mmol) was dissolved in a mixed solution of 4mL tert-butanol and 1mL deionized water, and after complete dissolution, tert-butyl hypochlorite (0.51g, 4.70mmol) was added dropwise, and stirred at room temperature for 36h in the dark . Compound 8 was obtained as a white solid (0.53 g, 95.5%) after concentration under reduced pressure.
[0032] 1 H NMR (500HMz,D 2 O) δ3.64-3.58(m,4H),3.37-3.31(m,4H),3.07(s,6H),2.12-2.06(m,2H),1.41(s,6H); 13 C NMR (126MHz, D 2 O) δ176.7, 155.4, 66.3, 61.7, 59.3, 50.9, 44.0, 36.4, 21.2, 20.9. HRMS calcd.for C 12 h 22 N 3 o 5 SCl[M+Na] + 378.0866,found: 378.0864.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com