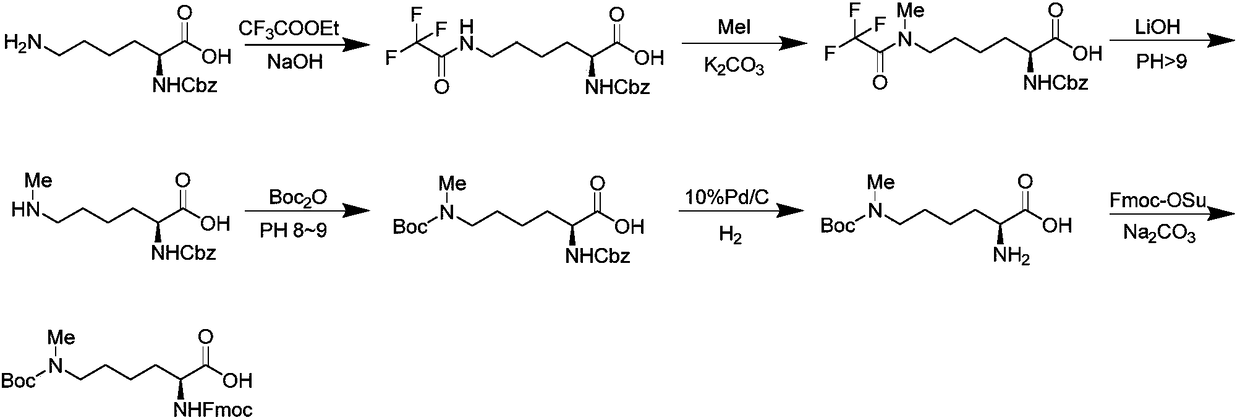
Preparation method of N epsilon-tert-butoxycarbonyl-N alpha-fluorenylmethoxycarbonyl-N epsilon-methyl-lysine
A technology of fluorene methoxycarbonyl and tert-butoxycarbonyl, applied in the field of organic synthesis, can solve problems such as being unsuitable for scale-up production, and achieve the effects of mild reaction conditions and concise synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
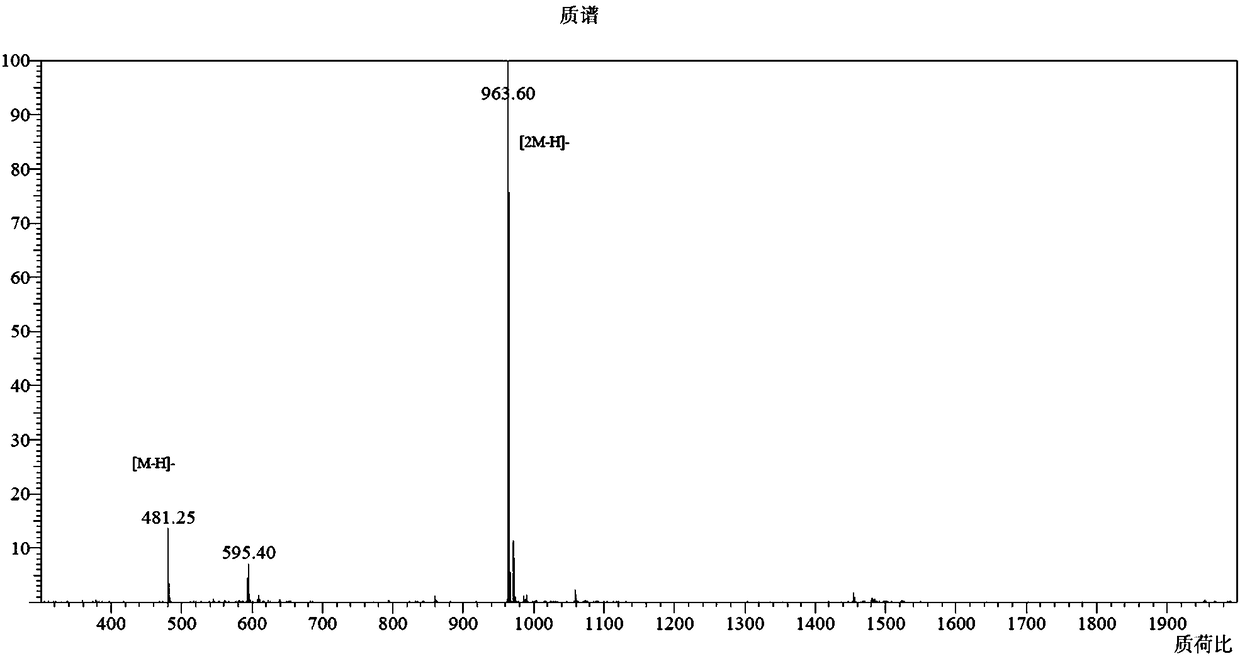
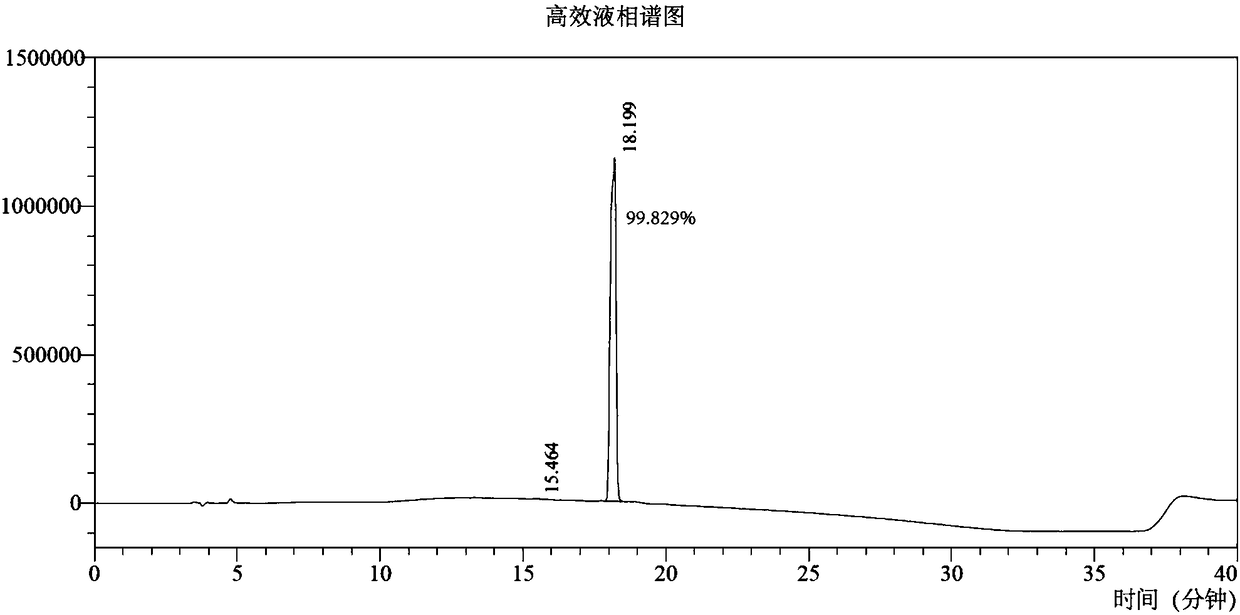
Image
Examples
Embodiment 1
[0025] In a 5.0L glass reaction flask, add Nα-fluorenylmethoxycarbonyl-lysine hydrochloride (200g, 0.5mol), N, N-diisopropylethylamine (DIEA) (258g, 2.0mol) successively and methanol (2.0 L). Stir at room temperature until clarification, at 0°C, slowly add trityl chloride (278g, 1.0mol) in tetrahydrofuran (1.0L) dropwise to the reaction solution, add about 1.0h, continue to react at 0°C for 2.0h, then The reaction was carried out at room temperature for 6.0 h, and HPLC detection showed that the raw materials had reacted completely. Concentrate under reduced pressure to remove the solvent, the crude product is separated with methanol and n-hexane, the methanol phase is washed 6-8 times with n-hexane, the methanol phase is concentrated, the crude product is extracted with ethyl acetate, washed with saturated brine, and the organic phase is evaporated to dryness to obtain a gray Crude solid.
[0026] The above gray solid was dissolved in 1.5L of methanol, 50ml (0.62mol) of 37% ...
Embodiment 2
[0030] In a 5.0L glass reaction flask, Nα-fluorenylmethoxycarbonyl-lysine hydrochloride (200g, 0.5mol), DIEA (387g, 3.0mol) and methanol (2.0L) were added sequentially. Stir at room temperature until clarification, at 0°C, slowly add trityl chloride (361g, 1.3mol) in tetrahydrofuran (1.5L) dropwise to the reaction solution, add about 1.0h, continue to react at 0°C for 2.0h, then The reaction was carried out at room temperature for 6.0 h, and HPLC detection showed that the raw materials had reacted completely. Concentrate under reduced pressure to remove the solvent, the crude product is separated with methanol and n-hexane, the methanol phase is washed 6-8 times with n-hexane, the methanol phase is concentrated, the crude product is extracted with ethyl acetate, washed with saturated brine, and the organic phase is evaporated to dryness to obtain a gray Crude solid.
[0031] The above gray solid was dissolved in 1.5L methanol, 50ml (0.62mol) of 37% formaldehyde solution and s...
Embodiment 3
[0035] In a 5.0L glass reaction flask, Nα-fluorenylmethoxycarbonyl-lysine hydrochloride (200g, 0.5mol), DIEA (387g, 3.0mol) and methanol (2.0L) were added sequentially. Stir at room temperature until clear. At 0°C, slowly add 2-chlorotrityl chloride (406g, 1.3mol) in tetrahydrofuran (1.0L) dropwise to the reaction solution. After about 1.0h, continue to react at 0°C for 2.0 h, and then reacted at room temperature for 6.0 h, and HPLC detection showed that the raw materials had reacted completely. Concentrate under reduced pressure to remove the solvent, the crude product is separated with methanol and n-hexane, the methanol phase is washed 6-8 times with n-hexane, the methanol phase is concentrated, the crude product is extracted with ethyl acetate, washed with saturated brine, and the organic phase is evaporated to dryness to obtain a gray Crude solid.
[0036] The above gray solid was dissolved in 1.5L of methanol, 60ml (0.74mol) of 37% formaldehyde solution and sodium cyano...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com