Cetagliptin salt crystal form and amorphism and preparation method and application thereof
An amorphous, crystal form technology, applied in the field of Shengglitin salt and its preparation, can solve the problems of lack of Shengglitin salt, crystal form, poor drugability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0115] Embodiment 1, the preparation method of the phosphate amorphous form of the compound of formula (I):
[0116] Dissolve 20 mg of the compound of formula (I) in 0.5 mL of methyl tert-butyl ether, then add phosphoric acid in an equimolar ratio to the compound of formula (I), stir and react at room temperature (25±2°C) for 12 hours, and collect the solid available.
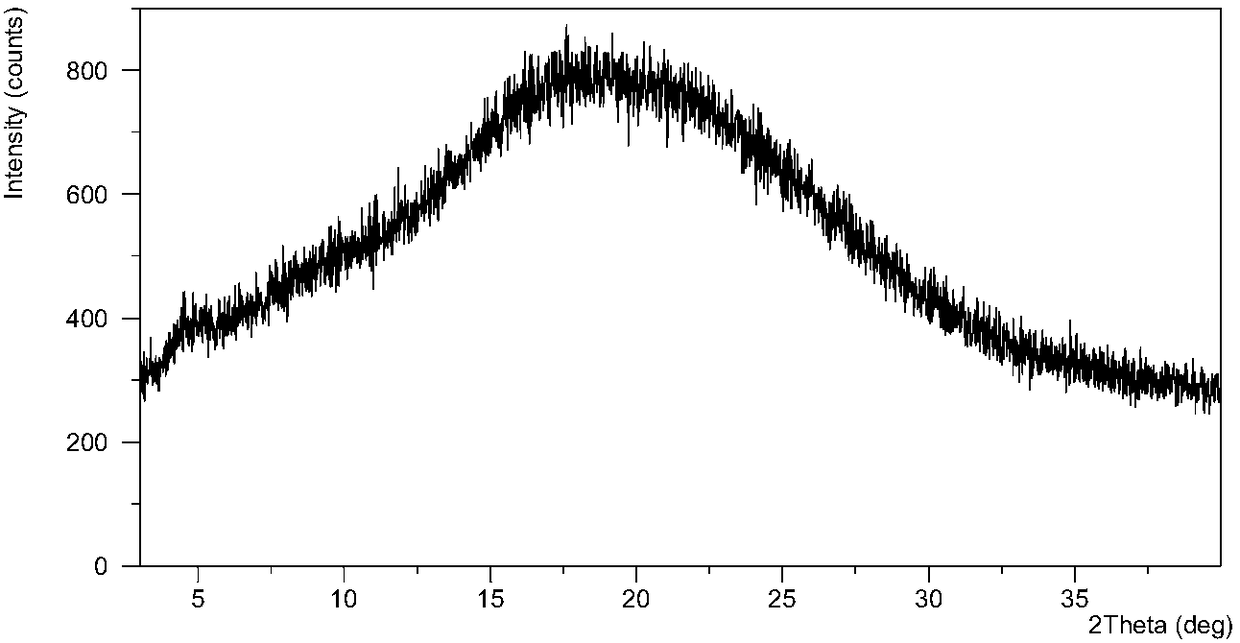
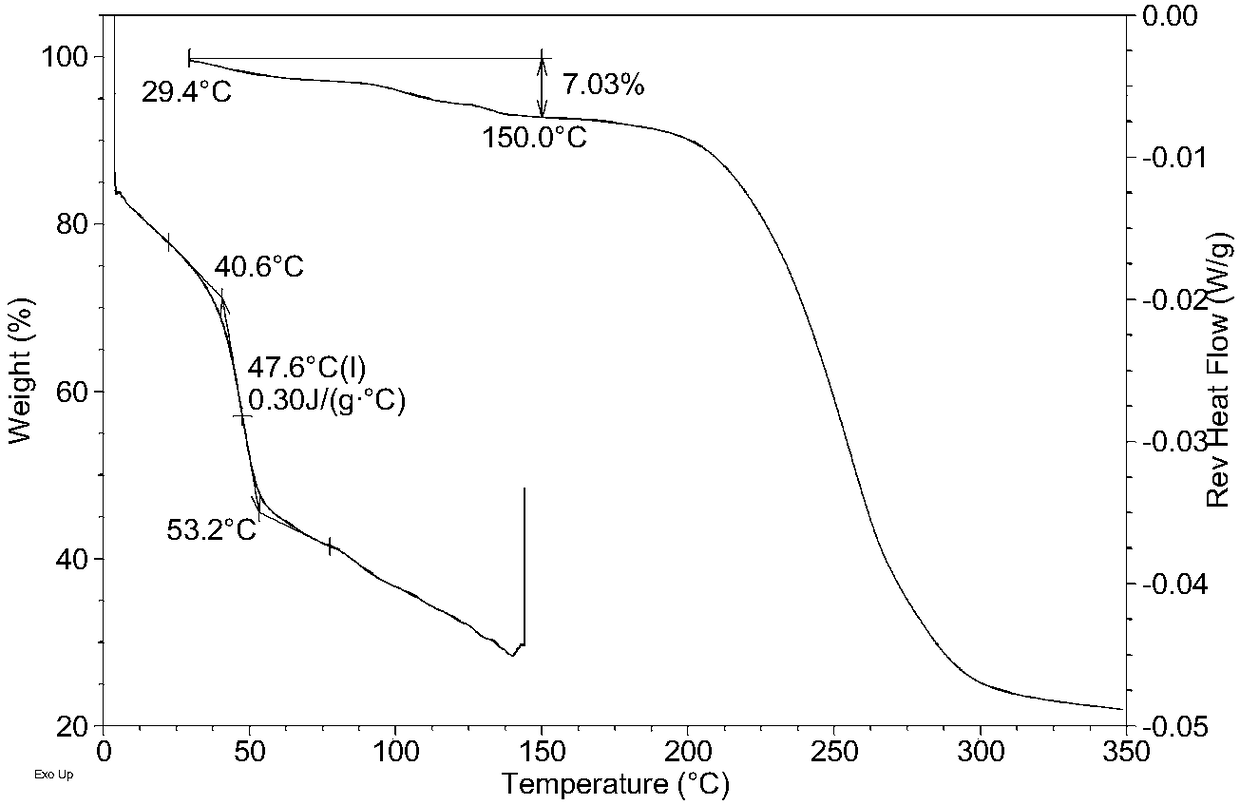
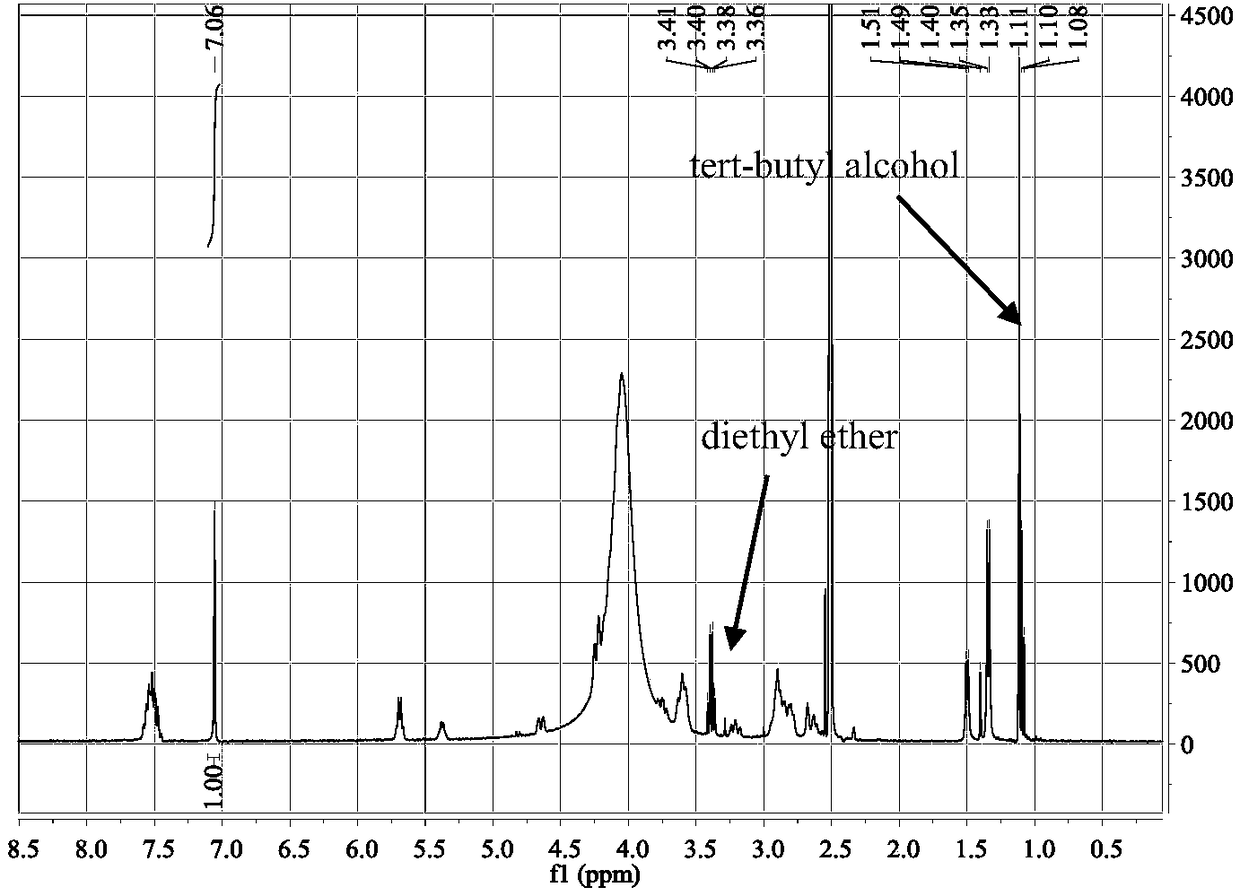
[0117] After testing, the resulting solid is an amorphous form of phosphate, and its XRPD pattern is as follows figure 1 , TGA diagram and DSC diagram such as figure 2 , 1H NMR as shown in image 3 . XRPD results indicated that the solid was amorphous. figure 2The middle TGA result showed that the sample had a weight loss of 7.0% when heated to 150°C, and the mDSC result showed that the glass transition temperature of the sample was 47.6°C (midpoint temperature). image 3 The 1H NMR (DMSO-d6) spectrum and the KF results (4.3%) in Table 7 below showed that the solid contained residual solvents diethyl eth...
Embodiment 2
[0127] Embodiment 2, the preparation method of the phosphate crystal form A of the compound of formula (I):
[0128] Dissolve the phosphate salt of the amorphous compound of formula (I) prepared in Example 1 in a mixed solvent of isoamyl alcohol and water with a volume ratio of 19:1, slowly volatilize the solution, and collect the solid to obtain it.
[0129] After testing, the obtained solid is crystal form A of phosphate, and its XRPD data is shown in Table 9 below, and its XRPD pattern is shown in Figure 4 , TGA diagram and DSC diagram as shown in Figure 5 . XRPD showed high crystallinity, TGA results showed that the sample had a weight loss of 6.4% when heated to 150°C, and DSC results showed that the sample had two endothermic peaks at 100.9°C and 132.7°C (peak temperature) before decomposition. Figure 6 XRPD characterization showed that phosphate form A transformed into phosphate form B after heating at 50 °C for 48 h.
[0130] Table 9
[0131]
Embodiment 3
[0132] Embodiment 3, the preparation method of the phosphate crystal form B of the compound of formula (I):
[0133] 15 mg of the phosphate salt of the amorphous compound of formula (I) prepared in Example 1 was dissolved in 1 ml of ethanol, and then evaporated slowly at room temperature (25±2° C.) to obtain a solid.
[0134] After testing, the obtained solid is crystal form B of phosphate, and its XRPD data is shown in Table 10, and its XRPD pattern is as follows Figure 7 , TGA graph and DSC graph such as Figure 8 , 1H NMR characterization results as Figure 9 shown. XRPD shows that the crystalline form has high crystallinity. The TGA results showed a 6.1% weight loss when the sample was heated to 150°C. DSC results showed that the sample had two endothermic peaks at 103.2°C and 133.5°C (peak temperature) before decomposition. The 1H NMR (DMSO-d6) spectrum shows that there is no signal peak of isopropanol, and combined with the weight loss of the crystal form B sample ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com