Erythromycin ethylsuccinate dry suspension and preparation method thereof
A technology of erythromycin ethylsuccinate and dry suspension, which is applied in the direction of pharmaceutical formulations, medical preparations with no active ingredients, medical preparations containing active ingredients, etc., and can solve the problem of poor redispersibility and erythromycin ethylsuccinate To solve the problems of poor flocculation effect of plain dry suspension, to achieve the effect of improving clinical curative effect, rapid absorption in gastrointestinal tract, and improving compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Erythromycin ethylsuccinate dry suspension
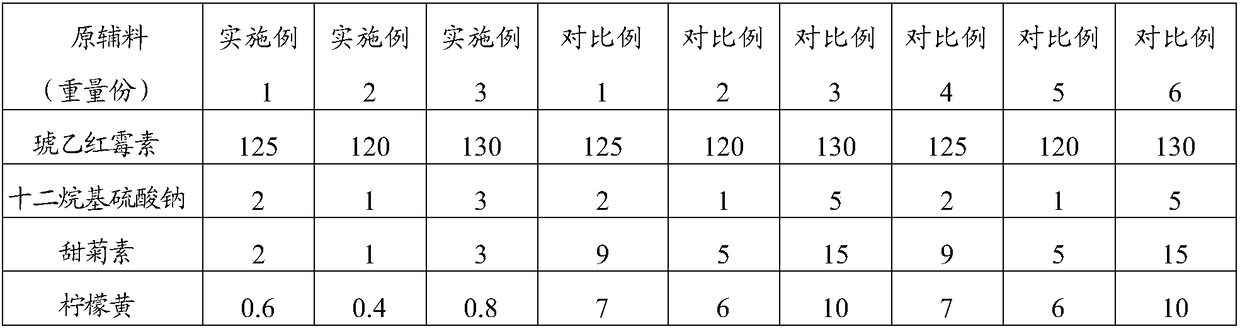
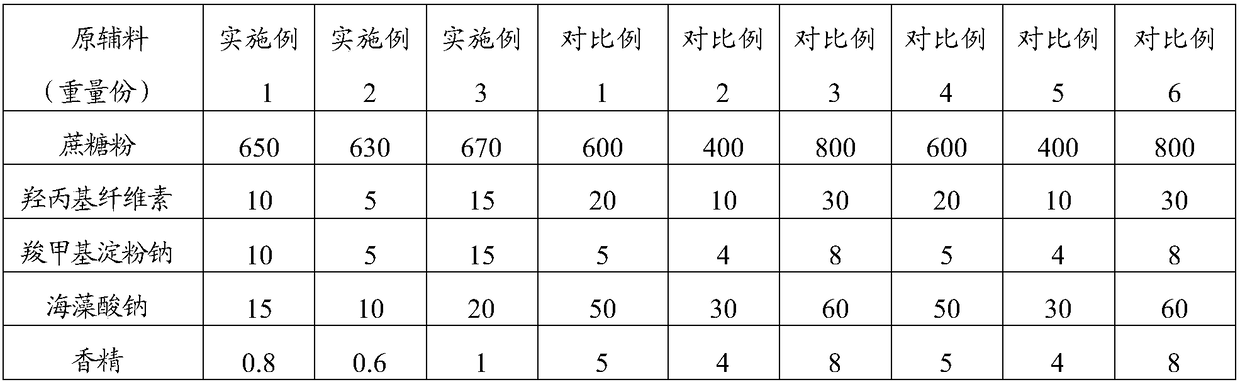
[0039] The raw material composition is shown in Table 1, where 1 part by weight = 1g.
[0040] The erythromycin ethylsuccinate dry suspension is prepared by the following method:
[0041] I. Prepare the components according to the weight ratio and crush them;
[0042] II. Take 1 / 3 of the weight of steviol, 1 / 2 of the weight of lemon yellow, sodium lauryl sulfate and erythromycin ethylsuccinate, 2 / 3 of the weight of sucrose powder, press Mix in equal increments, and granulate with a 20% ethanol aqueous solution (24ml) with a volume of 0.048 times the total mass of the mixed dry materials to obtain loose granules with hands that are clumped and loose and have burrs. And powder, part A, total 515g;
[0043] III. Take the remaining parts by weight of steviol, lemon yellow, sodium lauryl sulfate, erythromycin ethylsuccinate and sucrose powder, and the parts by weight of hydroxypropyl cellulose, sodium starch glycolate and sodium...
Embodiment 2
[0047] Example 2 Erythromycin ethylsuccinate dry suspension
[0048] The raw material composition is shown in Table 1, where 1 part by weight = 1g.
[0049] By the same method and steps as in Example 1, 940 bags of erythromycin ethylsuccinate dry suspension (0.1 g / bag based on erythromycin ethylsuccinate) were prepared.
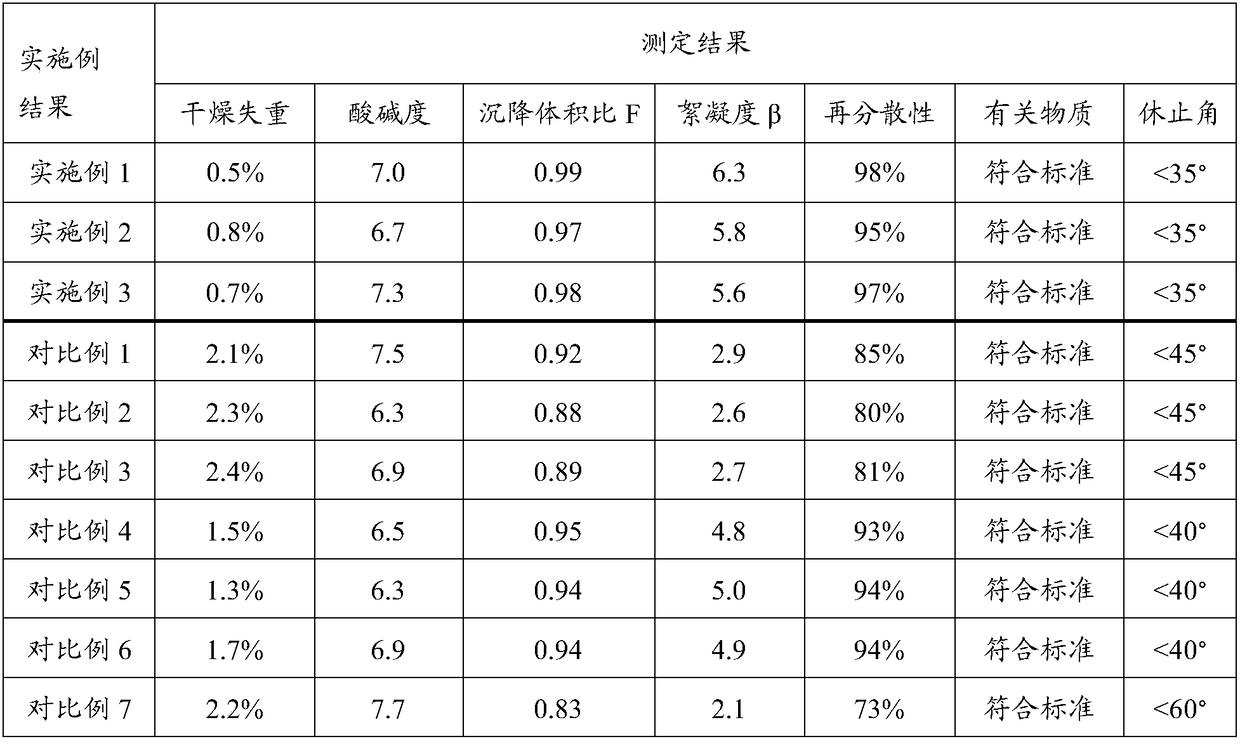
[0050] The dry suspension of erythromycin ethylsuccinate prepared in this example is light yellow particles and a small amount of powder. After adding an appropriate amount of water, the container is slightly shaken to form a uniform suspension with good taste, aroma and moderate sweetness.
Embodiment 3
[0051] Example 3 Erythromycin ethylsuccinate dry suspension
[0052] The raw material composition is shown in Table 1, where 1 part by weight = 1g.
[0053] Through the same method and steps as in Example 1, 1045 bags of erythromycin ethylsuccinate dry suspension (0.1 g / bag based on erythromycin ethylsuccinate) were prepared.
[0054] The dry suspension of erythromycin ethylsuccinate prepared in this example is light yellow particles and a small amount of powder. After adding an appropriate amount of water, the container is slightly shaken to form a uniform suspension with good taste, aroma and moderate sweetness.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com