Fe3O4 nano-particles with high dispersion stability in water phase, and preparation method thereof
A nanoparticle, highly dispersed technology, applied in the direction of nanotechnology, ferric oxide, iron oxide/iron hydroxide, etc., can solve the problems of physical and chemical properties change, blood vessel blockage, easy aggregation and sedimentation, etc., and achieve excellent affinity water effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
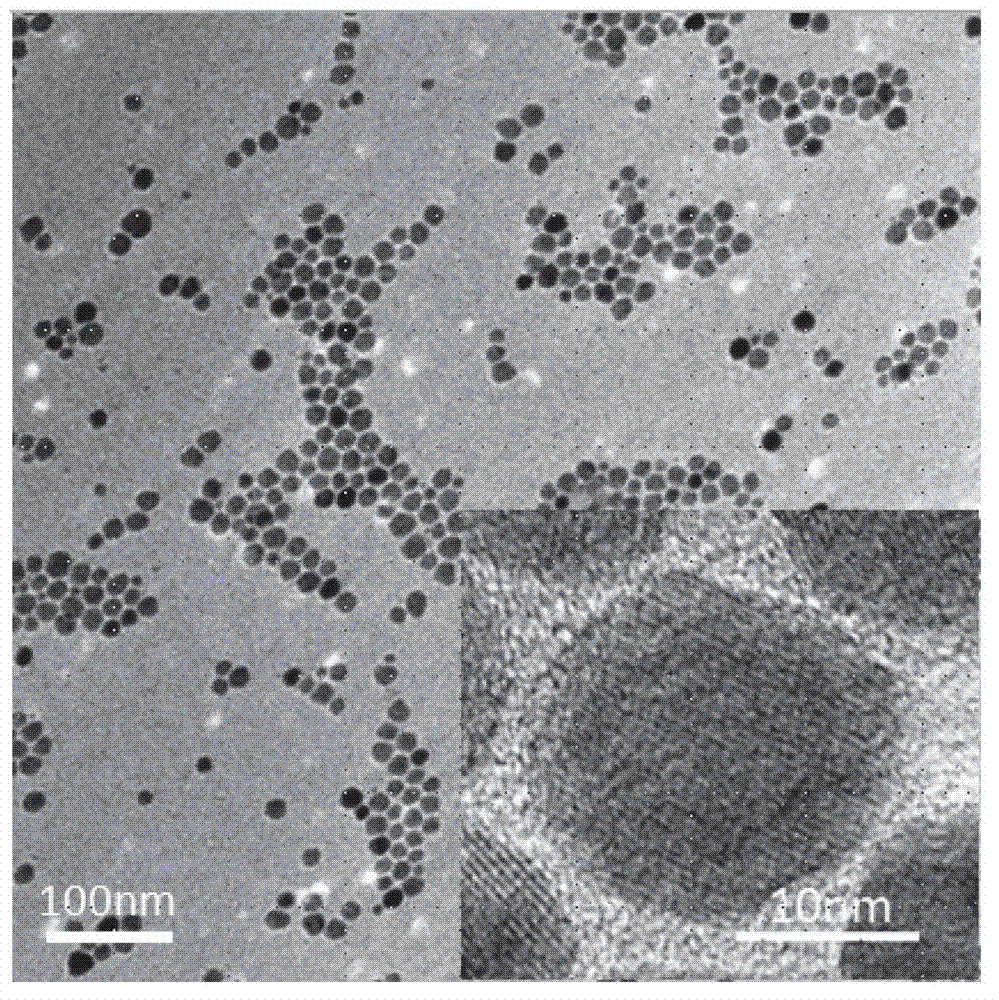
[0047] 1) Weigh 36.5g (120mmol) sodium oleate and 6.5g (40mmol) FeCl 3 Dissolve in a mixture of 140mL n-hexane, 80mL absolute ethanol and 60mL deionized water. After the solid is completely dissolved, transfer the solution into a 500mL three-necked flask, turn on the cooling water, heat the oil bath to 70°C, and stir mechanically for 4 hours. Then stop heating, transfer the reactant into a 500mL pear-shaped separatory funnel, take the upper oil phase, wash it with warm water at 50°C for several times, and place the final oil phase solution in a vacuum oven for one day to remove n-hexane , ethanol and water to obtain colloidal iron oleate.
[0048] 2) Weigh 9.0g (10mmol) of the ferric oleate prepared above and dissolve it in 60mL of 1-octadecene (ODE). After the ferric oleate is completely dissolved, transfer the solution into a 250mL three-necked flask, and drop Add 3.3mL (10mmol) oleic acid, under magnetic stirring and nitrogen protection, first raise the temperature to 110°...
Embodiment 2
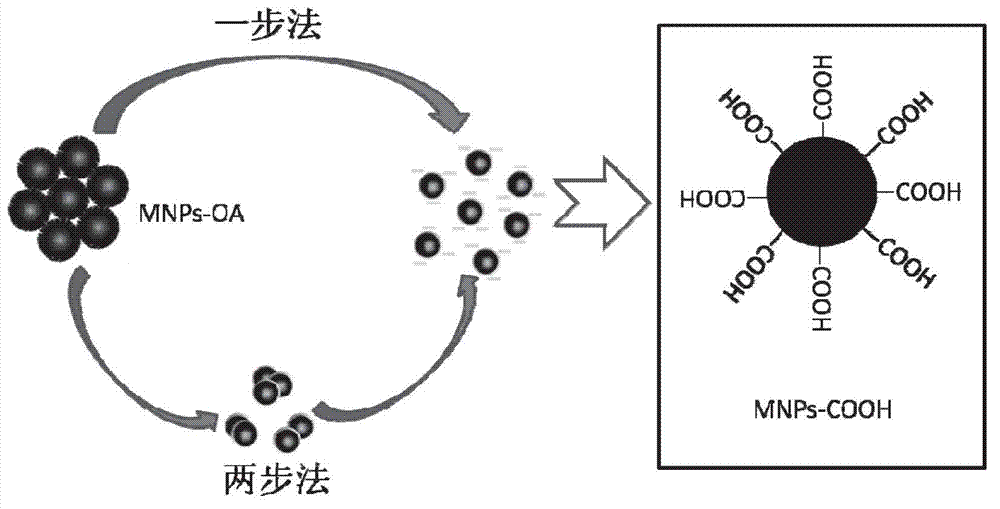
[0050] Take by weighing the prepared oil-soluble Fe of 120mg embodiment 1 3 o 4 Nanoparticles (MNPs-OA) were placed in a 100mL three-neck flask, ultrasonically dispersed with 20mL o-dichlorobenzene, and 20mL DMF solution containing 240mg citric acid monohydrate (CA) was added dropwise under ultrasonic, then sealed, and magnetically stirred at a certain speed , kept at 100° C. in an oil bath for 48 hours, after the reaction was completed, the solution was cooled to room temperature, washed several times by centrifugation with absolute ethanol, and the product was lyophilized into powder.
[0051] Wherein, the structural formula of citric acid monohydrate is as follows:
[0052]
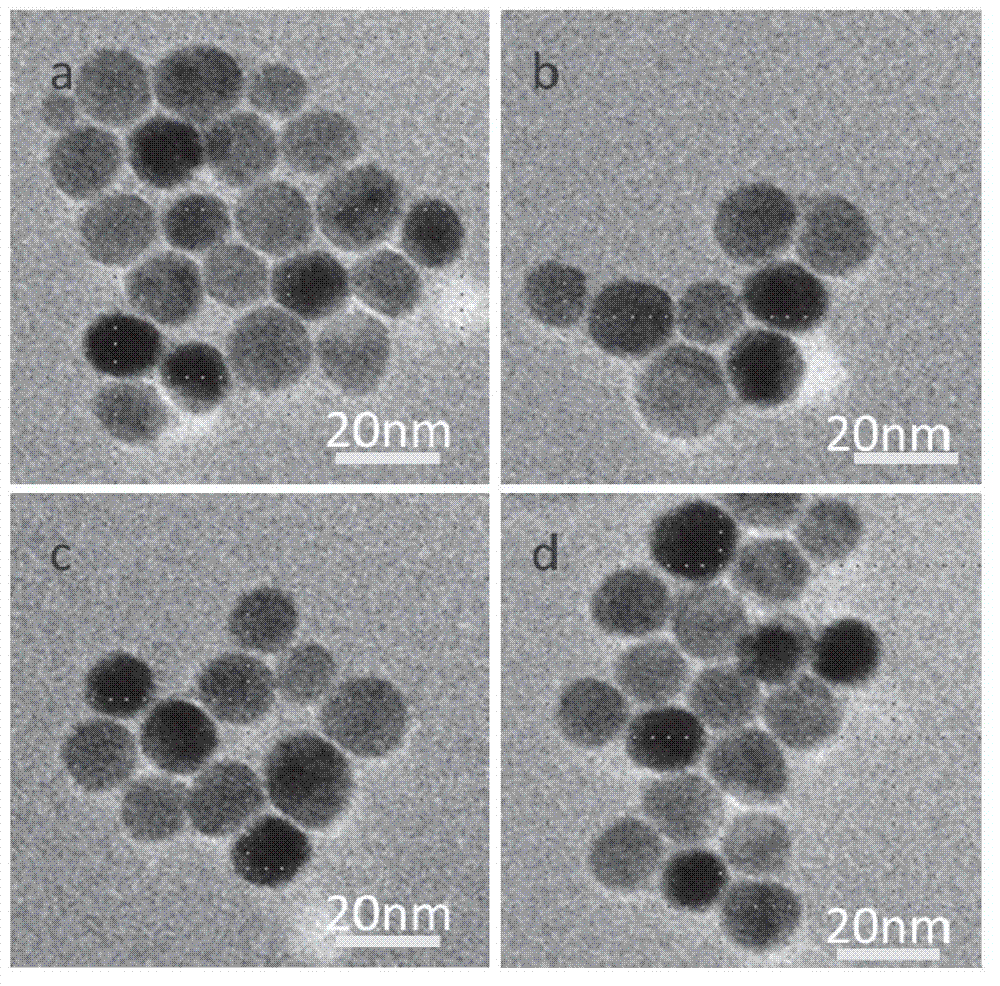
[0053] Depend on Figure 4 b It can be seen that the surface of the prepared Fe modified by citric acid 3 o 4 Nanoparticles (MNPs-CA) were dispersed in the water phase, but not in the oil phase, indicating that the prepared Fe 3 o 4 Nanoparticles are hydrophilic. The citric acid-modified wate...
Embodiment 3
[0055] Take by weighing the prepared oil-soluble Fe of 120mg embodiment 1 3 o 4 Nanoparticles (MNPs-OA) were placed in a 100mL three-necked flask, ultrasonically dispersed with 20mL o-dichlorobenzene, and 20mL DMF solution dissolved in 240mg butanetetracarboxylic acid (BTCA) was added dropwise under ultrasonic, then sealed, and magnetically Stir and keep warm in an oil bath at 100°C for 48 hours. After the reaction is completed, the solution is cooled to room temperature, centrifuged and washed several times with absolute ethanol, and the product is lyophilized into powder.
[0056]Wherein, the structural formula of butane tetracarboxylic acid is as follows:
[0057]
[0058] Depend on Figure 4 c shows that the prepared surface is modified by butane tetracarboxylic acid Fe 3 o 4 Nanoparticles (MNPs-BTCA) were dispersed in the water phase, but not in the oil phase, indicating that the prepared Fe 3 o 4 Nanoparticles are hydrophilic. The butane tetracarboxylic acid mo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com