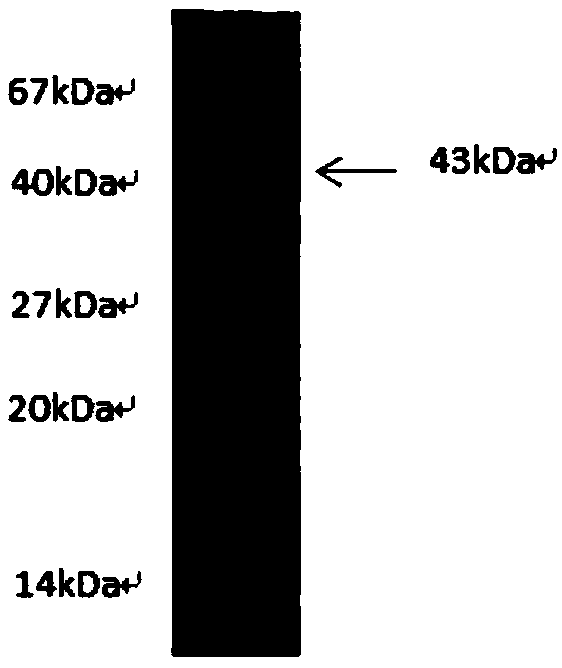Monoclonal antibody and preparation method thereof
A monoclonal antibody and antibody purification technology, which is applied in botany equipment and methods, biochemical equipment and methods, chemical instruments and methods, etc., can solve the problems of inability to produce influenza virus diagnostic reagents, etc., and achieve excellent reactivity and stable performance , the effect of high antigen titer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1 Antigen Preparation
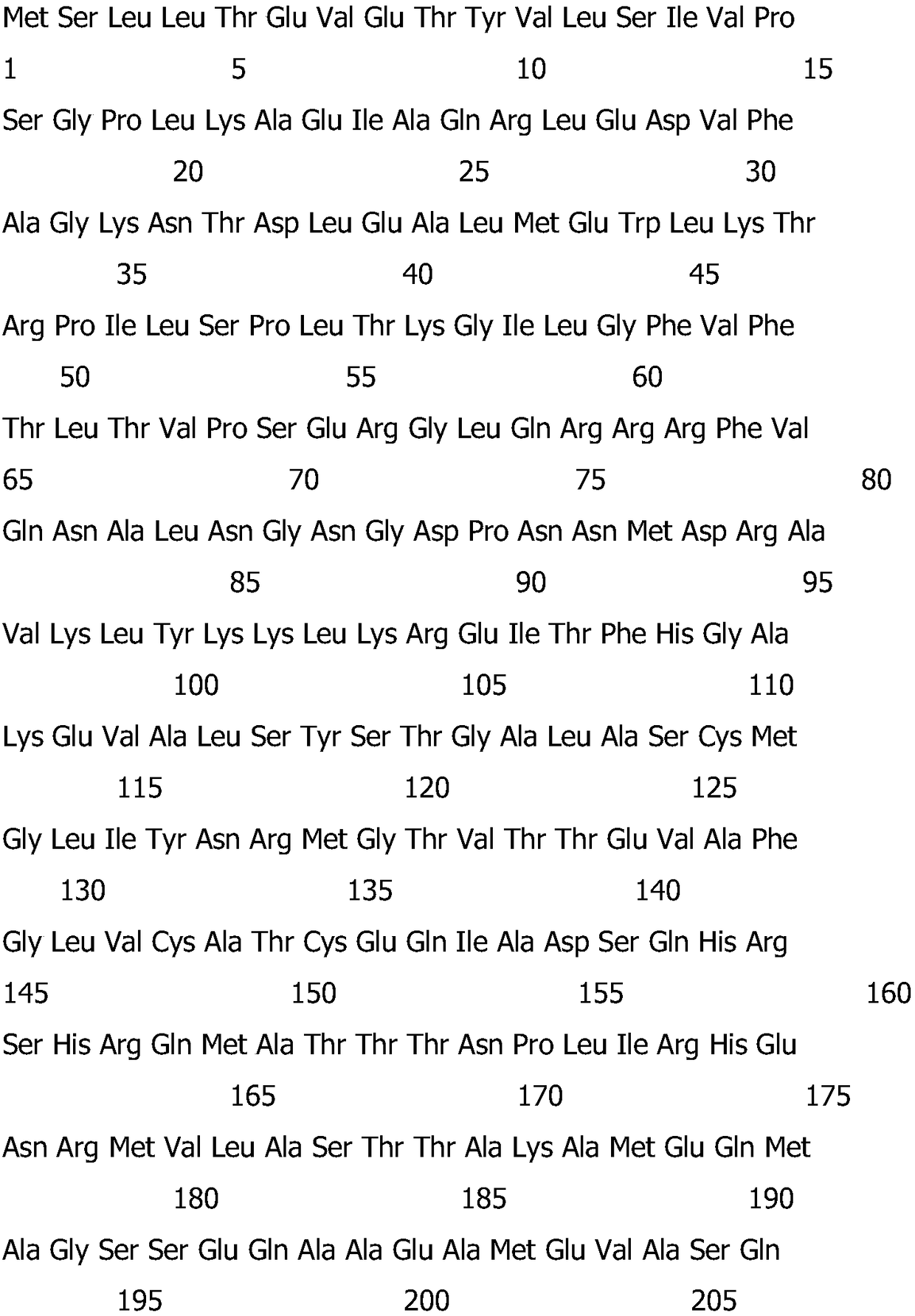
[0016] 1. Prepare the protein sequence matrix protein 1 [Influenza Avirus (A / PuertoRico / 8 / 1934 (H1N1))] of M1 gene, such as SEQ NO.1, numbered as C0131-Trx:
[0017]
[0018]
[0019] The preparation method is to insert the expression vector into the M1 gene, and insert the specified vector: pet32a
[0020] The M1 gene sequence is shown as SEQ NO.2:
[0021]
[0022]
[0023]
[0024]
[0025] Positive clone identification: After sequencing verification, the synthetic sequence is correct.
[0026] 2. Sample expression. Select 6 single clones from the transformation plate and inoculate 3ml of resistant medium respectively; culture to OD600nm0.5-0.6, add 0.5mM IPTG at 28°C to induce expression for 3.5 hours; collect the bacteria by centrifugation, sonicate, and detect the expression by SDS-PAGE. Analysis of small sample expression results: the protein is expressed in both the supernatant and the inclusion body, and solu...
Embodiment 2
[0029] Embodiment 2 animal immunization
[0030] 1 animal
[0031] Five Balb / C mice aged 5-8 weeks were immunized with the recombinant protein C0131-Trx and the viral protein mixture C0131-H9 provided by the customer, respectively.
[0032] 2 adjuvants
[0033] Freund's complete adjuvant was used for the first main injection, and Freund's incomplete adjuvant was used for subsequent booster injections. Both were fully mixed with an equal volume of antigen before injection.
[0034] 3 immunization methods. Multiple injections on the back. Main injection of 100ug antigen / rat, booster injection of 50ug antigen / rat.
[0035] immune cycle
[0036]
[0037]
[0038] 4. Antiserum detection. A small amount of blood was collected from the mouse tail vein to prepare antiserum. The titer of antiserum was detected by indirect ELISA method.
[0039]
[0040]
[0041] Reaction conditions: antigen coating: 37 degrees 2h
[0042] Closed: 37 degrees 1.5h
[0043] Serum ant...
Embodiment 3
[0047] Example 3 Cell Fusion and Subcloning
[0048] 1. Myeloma Cell Preparation
[0049] One week before fusion, revive SP2 / 0 cells and culture them to logarithmic phase normally.
[0050] 2. Splenocyte Preparation
[0051] The mice to be fused were selected and sacrificed by cervical dislocation on the day of fusion, the spleen was taken, and splenocytes were collected and counted according to the standard procedure.
[0052] 3. Cell Fusion
[0053] Mix myeloma cells and spleen cells at a ratio of 1:3-1:10, perform cell fusion operation according to the standard procedure, and then culture with HAT DMEM complete medium. Hybridoma cells can be seen 3 days after fusion, and replace them on the 7th day. 1 / 2HAT complete medium, change 1 / 2HT medium on the 8th day. Screening assays begin approximately 10 days after fusion.
[0054] Results of cell fusion: after fusion, cultured with HAT selective medium, observed under a microscope, many growing hybridoma cells were seen, pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com