Allene thiocyanide derivative and synthesis method thereof
A technology of allene thiocyanide and thiocyanide, which is applied in the direction of organic chemistry methods, chemical instruments and methods, organic chemistry, etc., can solve the problems of narrow substrate range, cumbersome operation, and low safety, and achieve easy preparation, The operation method is simple and the yield is good
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048]
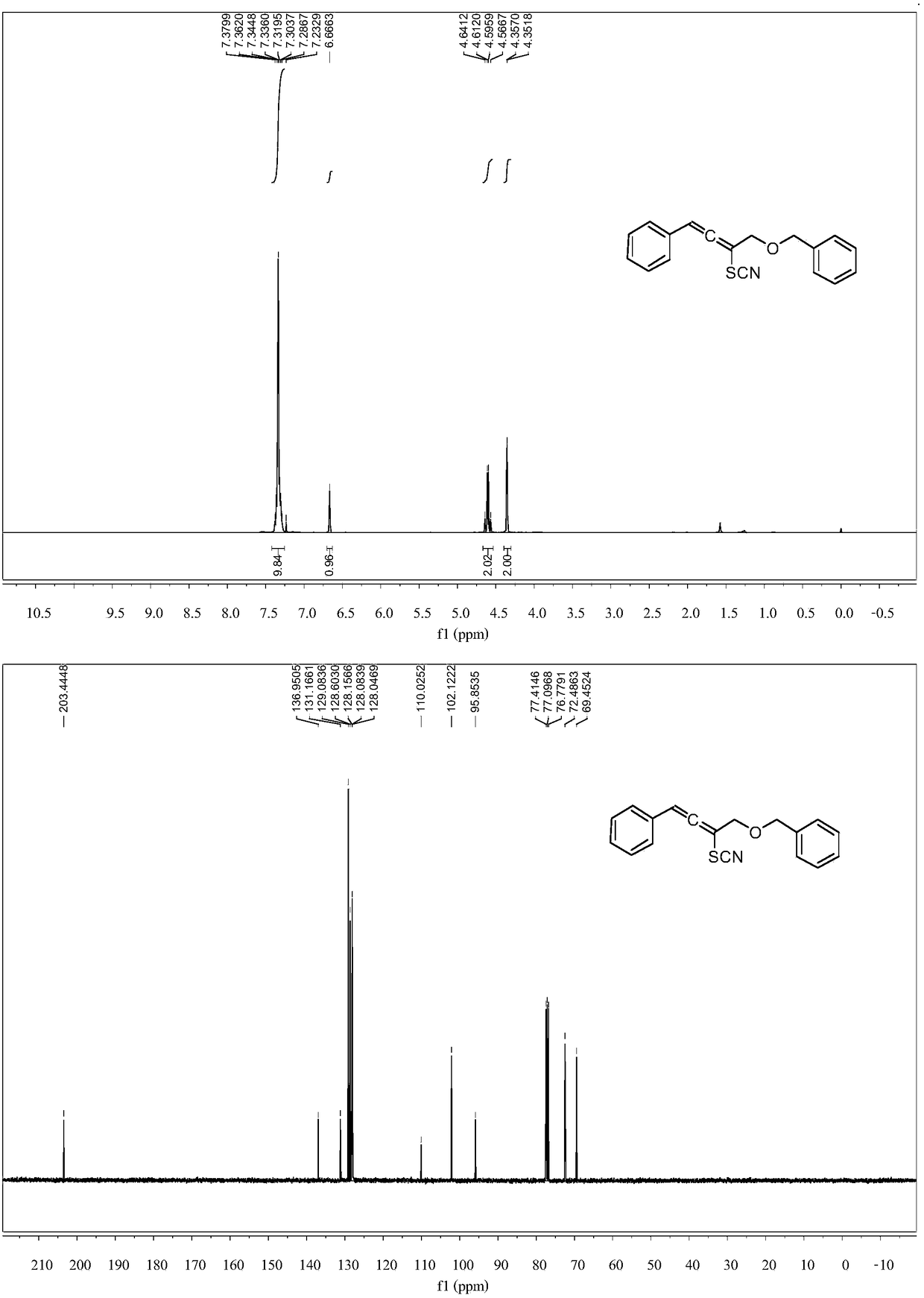
[0049] KBr (0.15mmol), AgSCF 3(0.15mmol) was added in acetonitrile (2mL), 4-(benzyloxy)-1-phenylbut-2-yn-1-amine (0.1mmol) was dissolved in acetonitrile (2mL), and then dissolved in acetonitrile ( 2 mL) of 4-(benzyloxy)-1-phenylbut-2-yn-1-amine (0.1 mmol) was added dropwise to the reaction system, and the reaction system was at room temperature. After the dropwise addition, stirred for 1 hour , and the solvent was removed under reduced pressure to obtain a crude product, which was subjected to column chromatography (ethyl acetate:petroleum ether=1:100~1:10) to obtain a pure product. Its structure is shown in formula (2-1). The yield was 80%. nuclear magnetic resonance 1 H NMR, 13 C NMR spectrum as figure 1 shown. 1 HNMR (400MHz, CDCl3) δ7.40–7.26 (m, 10H), 6.67 (s, 1H), 4.60 (q, J=11.7Hz, 2H), 4.35 (d, J=2.1Hz, 2H). 13 C NMR (101MHz, CDCl 3 )δ 203.44, 136.95, 131.17, 129.08, 128.60, 128.16, 128.08, 128.05, 110.03, 102.12, 95.85, 72.49, 69.45.
[0050] HRMS...
Embodiment 2
[0052]
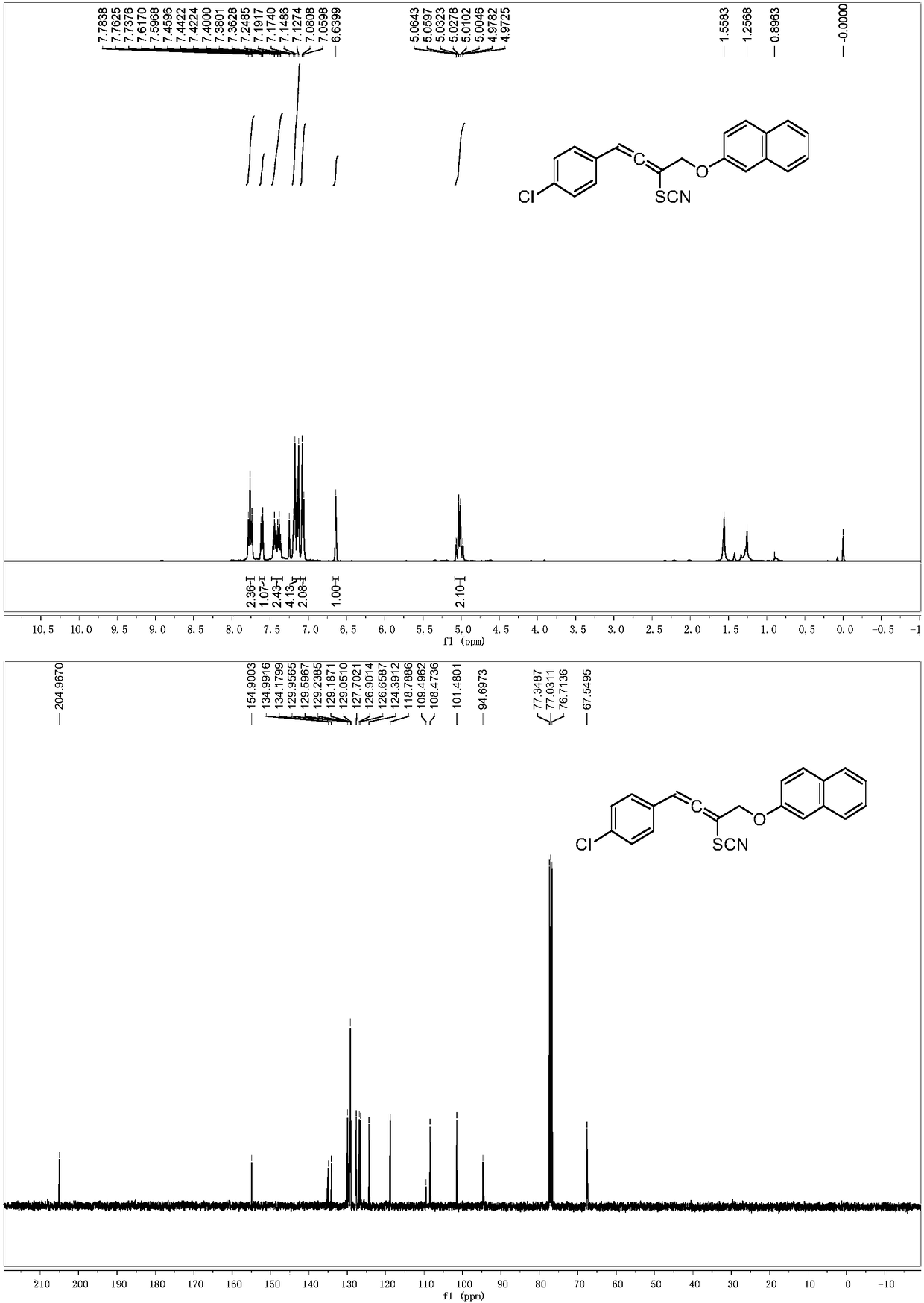
[0053] 1-(4-Chlorophenyl)-4-(naphthalen-2-yloxy)but-2-yn-1-amine was dissolved in acetonitrile (2 mL). KBr (0.15mmol), AgSCF 3 (0.15mmol), was added to acetonitrile (2mL), then 1-(4-chlorophenyl)-4-(naphthalene-2-yloxy)but-2-yne dissolved in acetonitrile (2.0ml) -1-Amine (0.1 mmol) was added dropwise to the reaction system, and the reaction system was at room temperature. After the dropwise addition, the reaction system was stirred for 1 hour, and the solvent was removed under reduced pressure to obtain a crude product. The crude product was subjected to column chromatography (ethyl acetate:petroleum ether=1:100~1:10) to obtain a pure product. Its structure is shown in formula (2-2). The yield was 81%. nuclear magnetic resonance 1 HNMR, 13 C NMR spectrum as figure 2 shown. 1 H NMR (400MHz, CDCl3) δ7.76 (t, J = 9.3Hz, 2H), 7.61 (d, J = 8.1Hz, 1H), 7.41 (dt, J = 14.9, 6.9Hz, 2H), 7.16 (dd ,J=17.9,7.8Hz,4H),7.07(d,J=8.4Hz,2H),6.64(s,1H),5.02(qd,J=12.8,2.1Hz,2...
Embodiment 3
[0056]
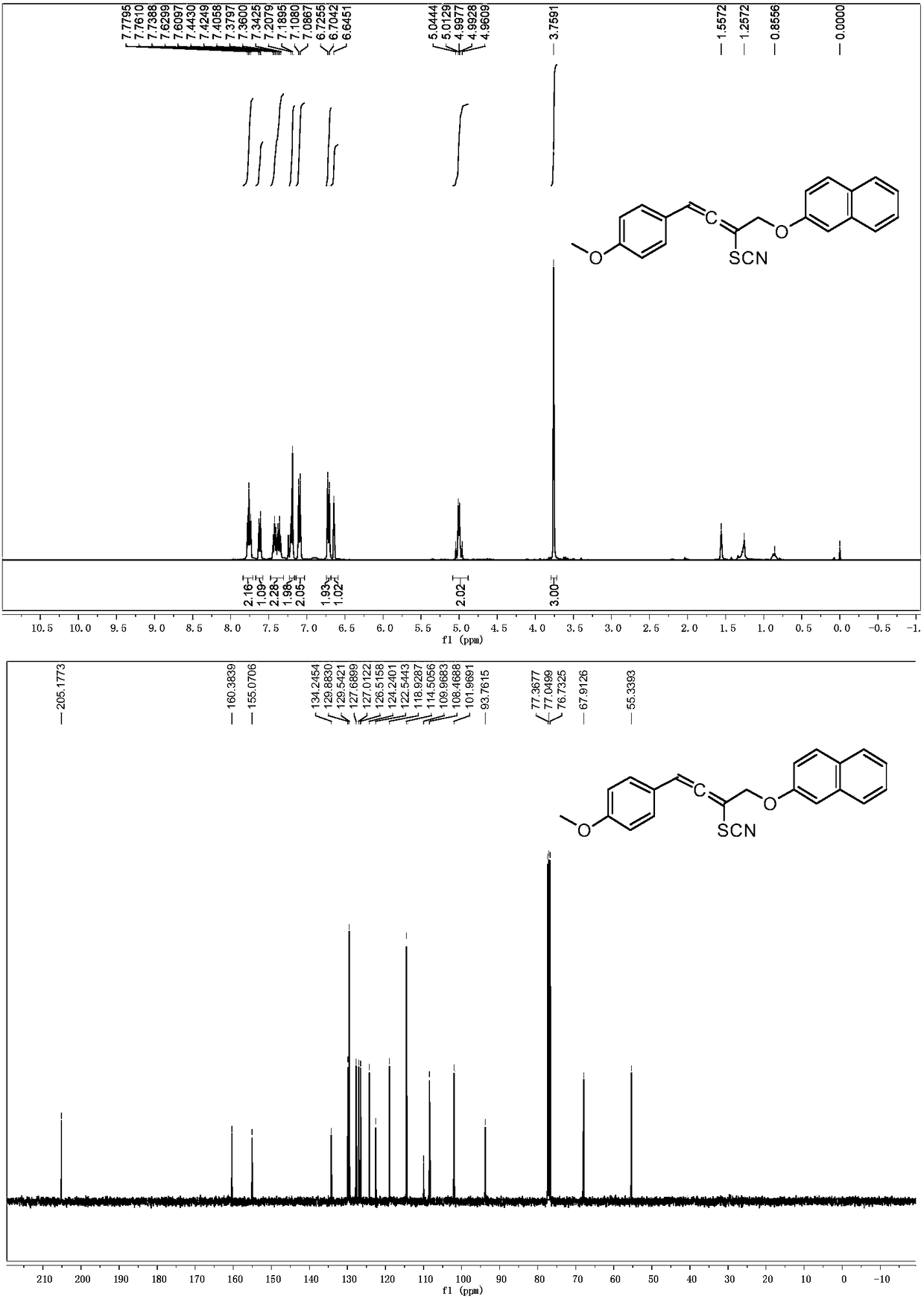
[0057] 1-(4-Methoxyphenyl)-4-(naphthalen-2-yloxy)but-2-yn-1-amine was dissolved in acetonitrile (2 mL). KBr (0.15mmol), AgSCF 3 (0.15mmol), was added to acetonitrile (2mL), then 1-(4-chlorophenyl)-4-(naphthalene-2-yloxy)butan-2 dissolved in (2.0ml) in acetonitrile -Alkyne-1-amine (0.1 mmol) was added dropwise to the reaction system, and the reaction system was at room temperature. After the dropwise addition was completed, it was stirred for 1 hour, and the solvent was removed under reduced pressure to obtain a crude product. The crude product was subjected to column chromatography (ethyl acetate:petroleum ether=1:100~1:10) to obtain a pure product. Its structure is shown in formula (2-3). The yield was 82%. nuclear magnetic resonance 1 HNMR, 13 C NMR spectrum as image 3 shown. 1 H NMR (400MHz, CDCl3) δ7.75 (dd, J = 8.0, 5.7Hz, 2H), 7.59 (d, J = 8.0Hz, 1H), 7.49–7.31 (m, 2H), 7.26–7.04 (m, 3H), 6.80(dd, J=7.2, 3.9Hz, 2H), 6.74(s, 1H), 6.65(s, 1H), 4.99(qd,...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap