Rare earth complex with near infrared pH intensity and life response
A technology of rare earth complexes and rare earth ions, which can be applied to organic compounds of group 3/13 without C-metal bonds, compounds containing elements of group 3/13 of the periodic table, organic chemistry, etc., which can solve the problem of short life and limited applications , Low luminous quantum yield and other issues, to achieve the effect of near-infrared intensity imaging and lifetime imaging
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The synthesis of embodiment 1 complex 1:
[0038]
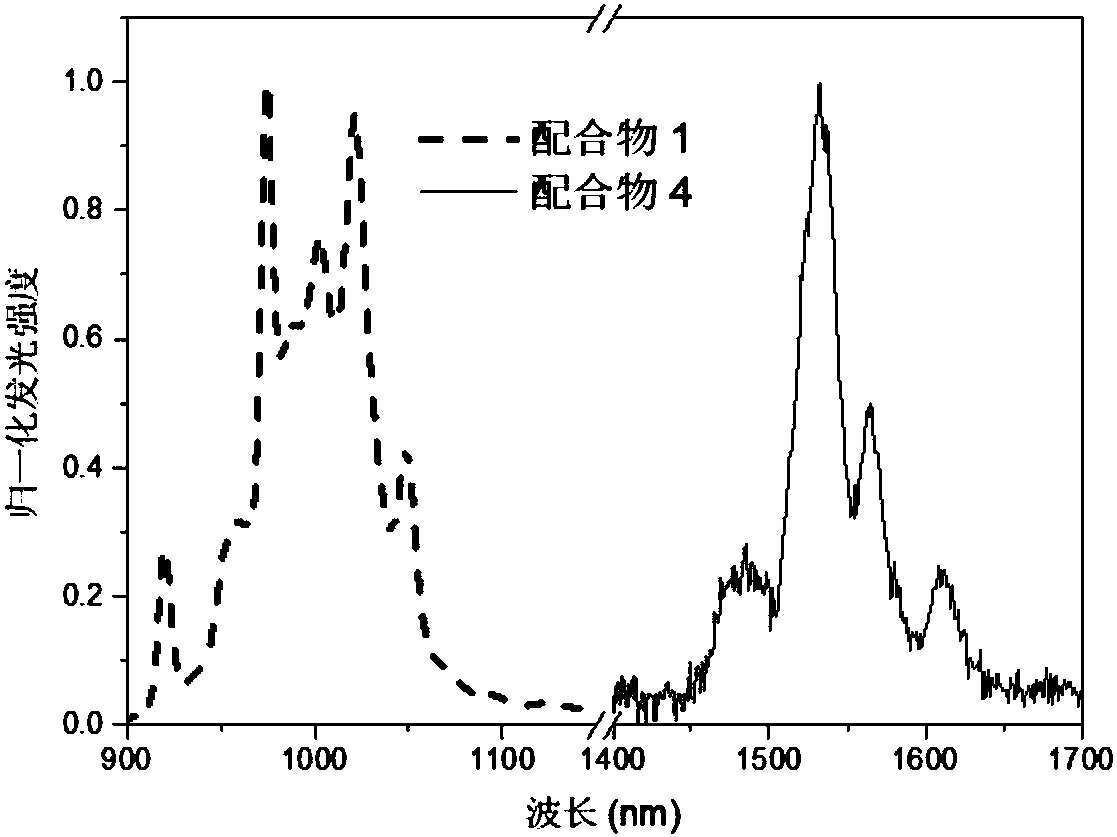
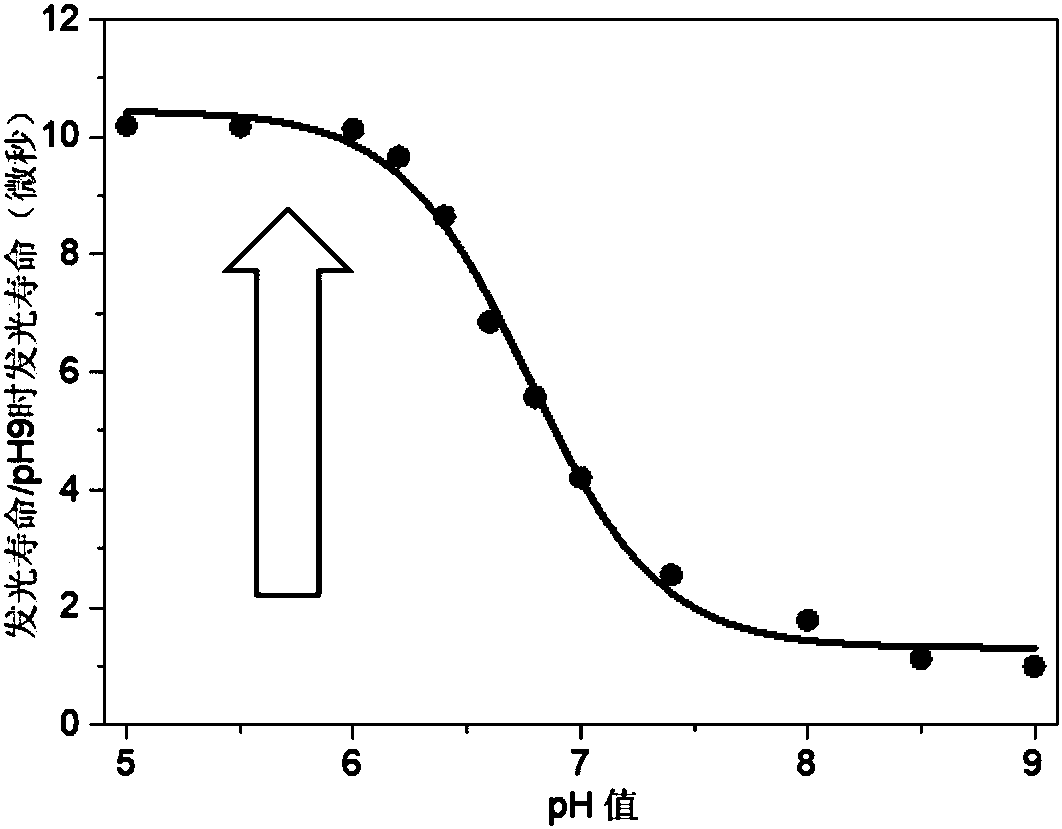
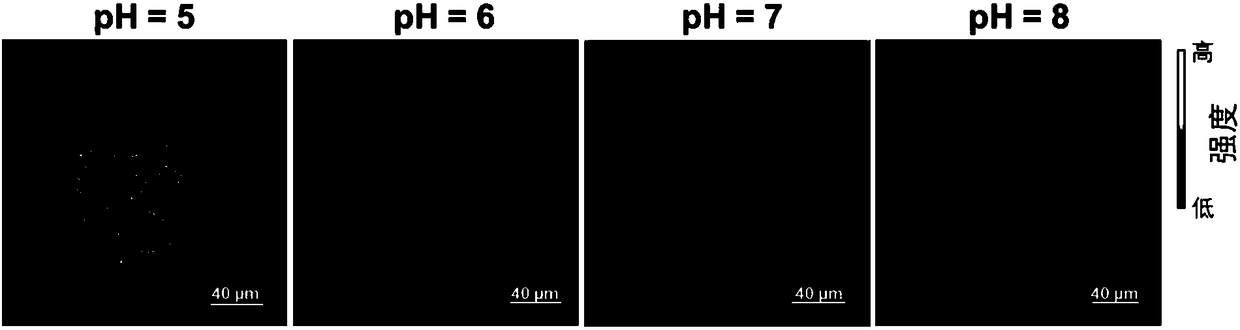
[0039] The first intermediate product was obtained by mixing the corresponding fluorinated tetrapyrrole ligand and ytterbium acetylacetonate salt in trichlorobenzene and reacting at 200° C. under nitrogen atmosphere. The resulting first intermediate and the corresponding deuterated The tripod ligand was reacted in chloroform to obtain a second intermediate product. Then react the second intermediate product in sulfuric acid to obtain complex 1. For the location of the main emission peak, see figure 1 : 920nm, 950nm, 980nm, 1000nm, 1020nm, 1050nm; main excitation peak position: 418 nm, 560nm, 620nm; lifetime: about 170μs.
Embodiment 2
[0040] The synthesis of embodiment 2 complex 2:
[0041]
[0042] The first intermediate product was obtained by mixing the corresponding fluorinated tetrapyrrole ligand and ytterbium acetylacetonate salt in trichlorobenzene and reacting at 200° C. under nitrogen atmosphere. The resulting first intermediate and the corresponding deuterated The tripod ligand was reacted in chloroform to obtain a second intermediate product. Then the second intermediate product was hydrolyzed by potassium hydroxide in methanol and tetrahydrofuran to obtain complex 2. Main emission peak position: 920nm, 950nm, 980nm, 1000nm, 1020nm, 1050nm; main excitation peak position: 416nm, 546nm, 592nm; lifetime: about 150μs.
Embodiment 3
[0043] The synthesis of embodiment 3 complex 3:
[0044]
[0045] The first intermediate product was obtained by mixing the corresponding fluorinated tetrapyrrole ligand and ytterbium acetylacetonate salt in trichlorobenzene and reacting at 200° C. under nitrogen atmosphere. The resulting first intermediate and the corresponding deuterated The tripod ligand was reacted in chloroform to obtain a second intermediate product. Then react the second intermediate product in (trimethylsilyl)phosphite to obtain complex 3. Main emission peak position: 920nm, 950nm, 980nm, 1000nm, 1020nm, 1050nm Excitation main peak position: 416nm, 546nm, 592nm; lifetime: about 140μs.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com