Chloroquine cholesterol derivative and its preparation method and use
A technology of cholesterol derivatives, chloroquine cholesterol, which is applied in the field of chemical medicine, can solve problems that need to be studied, and achieve the effect of inhibiting proliferation and treating pulmonary fibrosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Synthesis of 2-(4-(7-chloroquine-4-amino)pentyl-(ethyl)aminoethyl)-cholesterol succinate (compound 1)
[0034]
[0035] Put cholesterol, succinic anhydride, and DMAP in an appropriate amount of dichloromethane in a molar ratio of 1:2.5:2.5, stir at room temperature for 48 hours, extract 5 times with 1mol / L HCl at the end of the reaction, and wash 5 times with 200mL water to remove Concentrate most of dichloromethane, add 100mL glacial acetic acid, remove all solvents to dryness, wash the product with a little glacial acetic acid several times in a Buchner's hole until white, dry at 60°C overnight to obtain cholesterol succinic acid Single fat.
[0036] After hydroxychloroquine sulfate is desalted and dried with NaOH solution, hydroxychloroquine, cholesterol succinic acid monoester, DMAP, EDCI (1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride ) According to the molar ratio of 1:1.2:1.2:1.2 ratio feeding reaction. First, dissolve hydroxychloroqui...
Embodiment 2
[0039] Embodiment 2 Compounds of the present invention inhibit rat fibroblast proliferation
[0040] Fibroblasts were isolated from rat lung tissue, and the 4-8 generation cells were used for experiments, and they were indeed fibroblasts identified by Vimentin (vimentin) immunofluorescent staining. The cells were cultured in DMEM medium containing 10% FBS (fetal bovine serum, purchased from GIBCO).
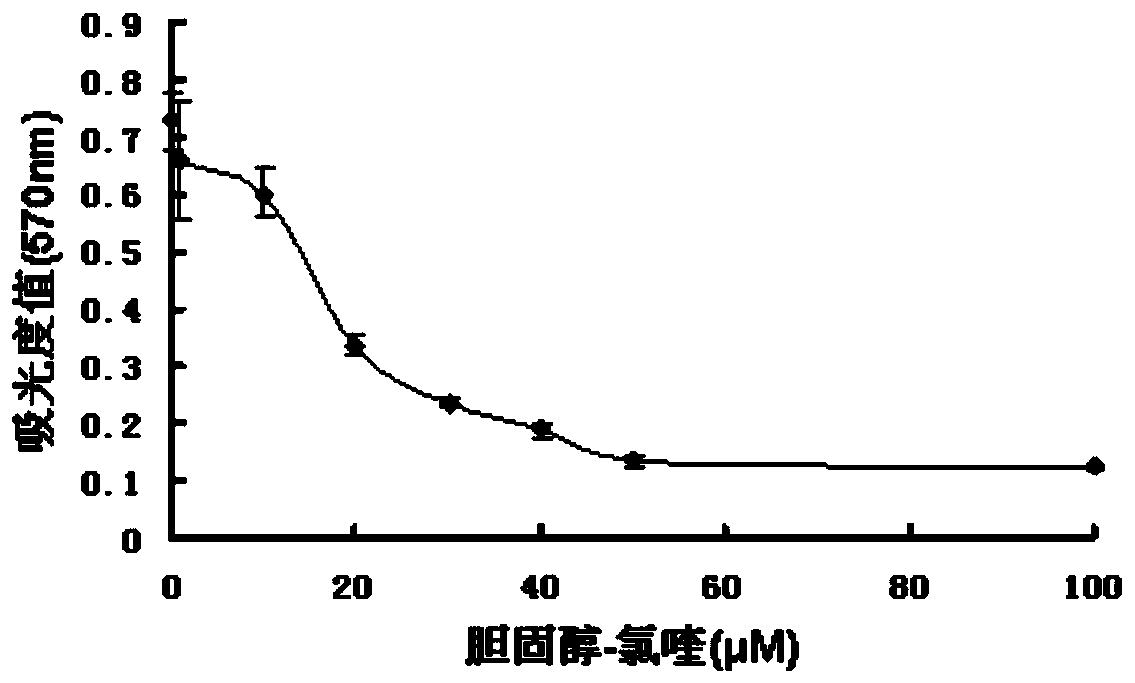
[0041]Inoculate 4,000 fibroblasts in each well of a 96-well plate, add compound 1 for treatment after adherence, divide into DMSO control group, set the concentration gradient from 1 to 100 μM, treat for 24 hours, add MTT (tetrazolium bromide) for incubation, and crystal violet dissolves The absorbance value was detected in DMSO (dimethyl sulfoxide) at a wavelength of 570 nm.
[0042] See the experimental results figure 1 Shown: Rat fibroblasts treated with different concentrations of compound 1, the absorbance value at 570nm wavelength at 24 hours, as the concentration increase...
Embodiment 3
[0043] Embodiment 3 compound 1 anti-pulmonary fibrosis animal experiment
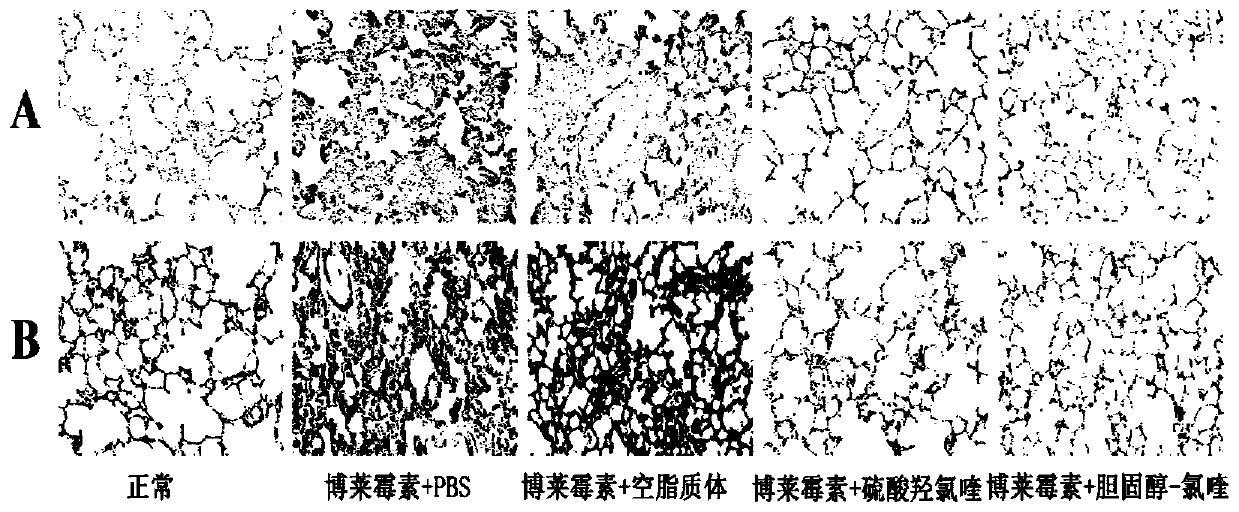
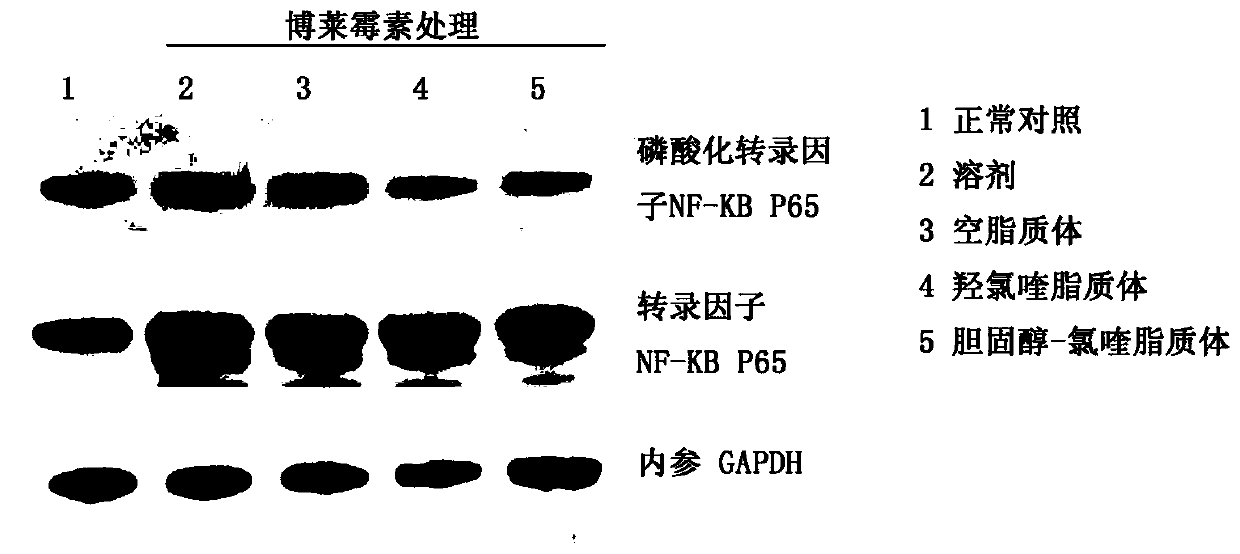
[0044] SD rats, female, with a body weight of about 250 grams, were given a single large dose of bleomycin (5 mg / kg body weight) in the rat's trachea for the modeling of pulmonary fibrosis, and the administration began the next day, and PBS (phosphate Buffer solution) control group, empty liposome control group, hydroxychloroquine sulfate control group (8mg / kg / day), compound 1 treatment group (20mg / kg / day). 15 rats per group were tested. Rats were sacrificed on the 7th day, 14th day, and 28th day, and the lung tissues were taken out, fixed in 4% paraformaldehyde, embedded in paraffin, sectioned, H&E (hematoxylin-eosin) staining and Masson staining (three color staining) to evaluate the therapeutic effect.
[0045] See the experimental results figure 2 Shown: Compound 1 and hydroxychloroquine sulfate are used as the animal experimental research results of anti-pulmonary fibrosis treatment drug, A is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com