Tissue engineering acellular vascular scaffold preparation method
A vascular stent and tissue engineering technology, applied in the field of tissue engineering decellularized vascular stent preparation, can solve the problems of disease transmission risk limitation and difficult implementation, and achieve the effects of reducing immune response, improving mechanical properties, and improving vascular mechanical properties.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
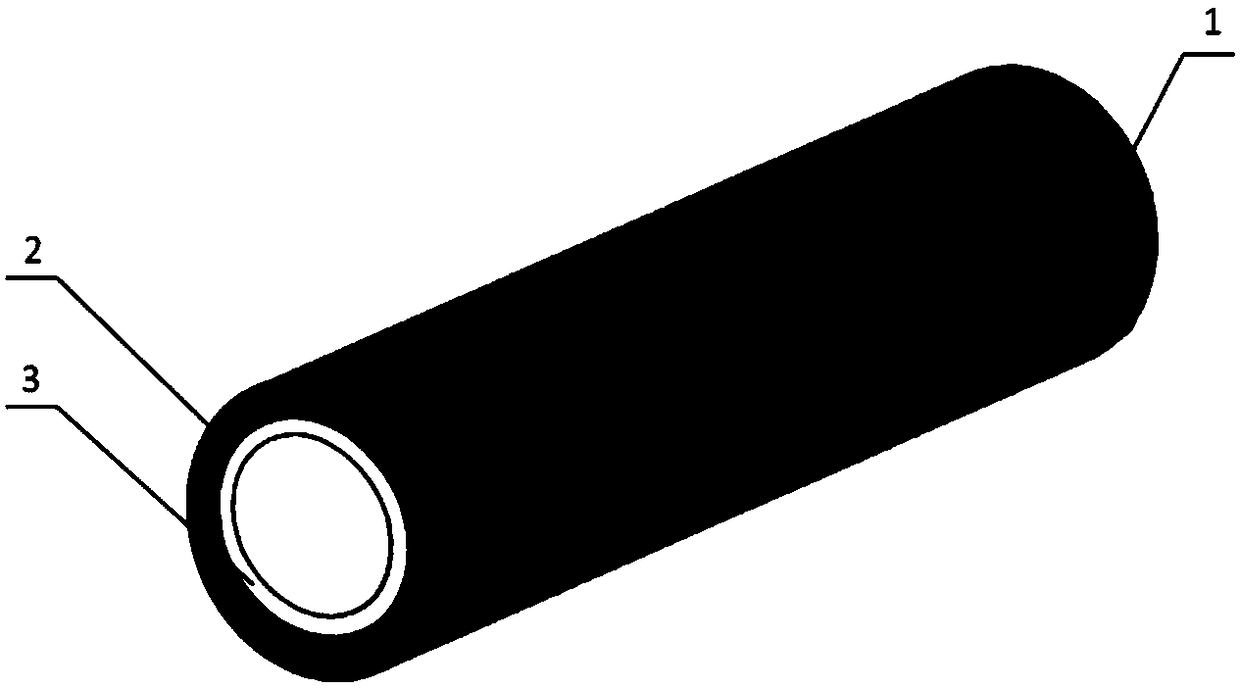
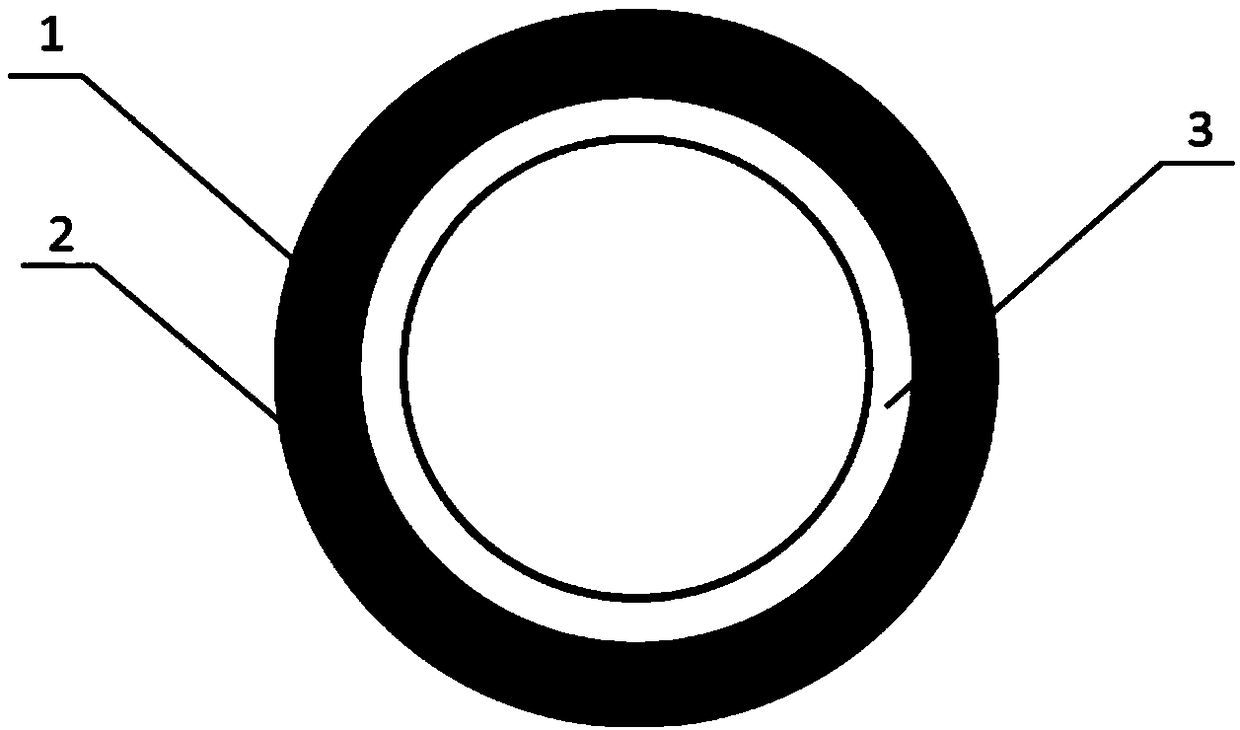
[0025] 1) Using biodegradable polymer materials as raw materials, wet spinning technology is used to prepare micron-scale or nano-scale ordered reticular vascular stents on medical silicone tubes. External helical winding, the angle between the fibers is 45°, the thickness of the mesh vascular stent layer is 600 μm, and the diameter of the vascular stent is 2mm; figure 1 It is a schematic diagram of the three-dimensional structure of decellularized blood vessels; figure 2 It is an enlarged schematic diagram of the cross-sectional structure of the decellularized vascular stent; specifically, dissolve 1 gram of PLCL in 10 mL of hexafluoroisopropanol, stir at room temperature until completely dissolved, connect a medical silicone tube with a diameter of 2 mm to a rotating motor, and inhale the PLCL solution In the syringe, the needle of the syringe is placed in an ethanol coagulation bath at a position 1 cm away from the silicone tube. Set the solution flow rate to 2ml / h, recei...
Embodiment 2
[0029] The preparation method of embodiment 2 is the same as that of embodiment 1, the only difference is that the preparation method of the spinning fiber skeleton is different: in this embodiment, the composite mandrel of melt spinning and medical silicone tube is prepared by using melt spinning equipment, and the melt spinning Wire equipment, including syringes, stainless steel single-tube needles and cylindrical receivers, in this embodiment, medical silicone tubes are sheathed with stainless steel rods to provide sufficient support to prevent the cylindrical structure from being squeezed and deformed; stainless steel syringes 1 are equipped with Spinning polymer melt; the polymer melt flows out from the all-stainless steel single-tube needle of the syringe, and rotates and translates with the cylindrical receiver, and the fine stream of polymer melt used for spinning is stretched and thinned, by adjusting the cylinder The angle of fiber arrangement can be adjusted by adjus...
Embodiment 3
[0032]Embodiment 3: embodiment 3 is similar to embodiment 2, and the difference is only that the preparation method of melt-spun fiber skeleton is different, takes by weighing 5.0g, and number average molecular weight is 80000PCL, places the sealing rust-free that fuser wraps In a steel syringe, heat at 200 °C for 1 h. Put a medical silicone tube with an outer diameter of 2mm and an inner diameter of 1mm on a stainless steel rod with a diameter of 1mm and connect it to the rotating motor. The distance between the syringe needle and the receiver is 5mm, the flow rate of the PCL melt is 0.1ml / h, and the rotation of the receiving rod The speed was set to 300r / min, the translational speed was set to 4mm / s, and the receiving device horizontally reciprocated to receive the fiber 20 times, and a composite mandrel of PCL melt-spun fiber and medical silicone tube with a fiber angle of 30° was prepared. The diameter of the spun fiber is 5 μm, and the thickness of the fiber skeleton is 4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com