A shape-controllable fe 3 o 4 Preparation methods of nanomaterials
A nanomaterial, fe3o4 technology, applied in the direction of nanotechnology, ferrous oxide, iron oxide/iron hydroxide, etc., can solve the problems of unfavorable industrialization development, low production efficiency, and complicated preparation of raw materials, and achieve non-magnetic Hysteresis, low cost, good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
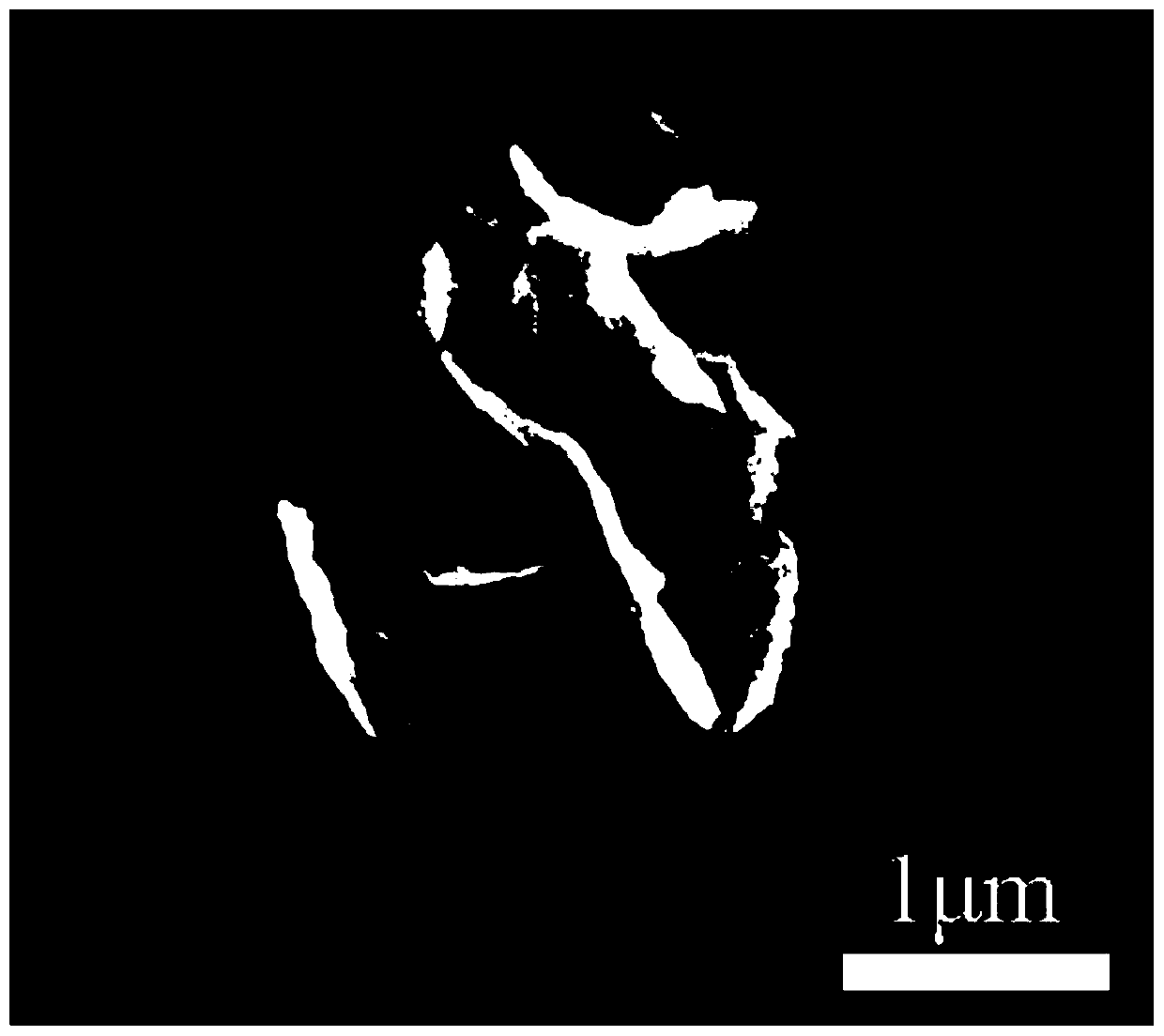
[0037] FeCl 3 ·6H 2 O and ethylene glycol were placed in a beaker, FeCl 3 ·6H 2 The mass volume ratio (mg / mL) of O and ethylene glycol is 30:1, then add urea, FeCl 3 ·6H 2 The molar ratio of O and urea is (mol) 4:17, then mechanically stir the mixture in the beaker to mix the above raw materials evenly, pour it into a three-necked flask and heat it under reflux, the temperature of reflux heating is 195°C, and the time of reflux heating is 5min , continue to feed nitrogen during the reflux heating process, then cool to room temperature, and use ethanol to wash centrifugally to obtain Fe 3 o 4 Precursor. figure 1 It is the Fe that the present invention obtains when the time of reflux heating is 5min 3 o 4 SEM images of the precursors. Finally, the precursor nanosheets were calcined in a high-temperature furnace, and the temperature was maintained at 500 °C for 3 h, and nitrogen protection was introduced.
[0038] The final product is Fe with a size of 20 nm 3 o 4 Nan...
Embodiment 2
[0040] FeCl 3 ·6H 2 O and ethylene glycol were placed in a beaker, FeCl 3 ·6H 2 The mass volume ratio (mg / mL) of O and ethylene glycol is 30:1, then add urea, FeCl 3 ·6H 2 The molar ratio of O and urea is (mol) 4:3, then mechanically stir the mixture in the beaker to mix the above raw materials evenly, pour it into a three-necked flask and heat it under reflux, the temperature of reflux heating is 195°C, and the time of reflux heating is 20min , continue to feed nitrogen during the reflux heating process, then cool to room temperature, and use ethanol to wash centrifugally to obtain Fe 3 o 4 Precursor nanosheets. Finally, the precursor nanosheets were calcined in a high-temperature furnace, and the temperature was maintained at 500 °C for 3 h, and nitrogen protection was introduced.
[0041] The final product is Fe with a size of 3.5 μm 3 o 4 Nanosheets, the product morphology is as follows figure 2 As shown, the saturation magnetization is 70emu / g, and the remanenc...
Embodiment 3
[0043] The difference between this embodiment and embodiment 2 is that FeCl 3 ·6H 2 The molar ratio of O and urea is (mol) 4:11, all the other are identical with embodiment 2.
[0044] The final product is Fe with a size of 2.5 μm 3 o 4 Nano-flower-like particles, the product appearance is as follows image 3 As shown, the saturation magnetization is 73emu / g, and the remanence and coercive force are approximately zero.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com