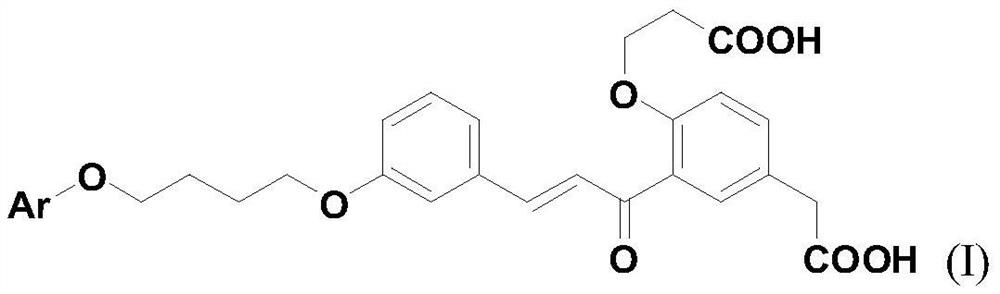
A kind of dicarboxychalcone compound and its application in the preparation of anti-inflammatory drugs
A technology of chalcones and methylchalcones, which is applied in the preparation of oxygen-containing compounds, organic compounds, anti-inflammatory agents, etc., can solve the problem of selective antagonist research starting late and achieve obvious ear swelling , the effect of suppressing auricle swelling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
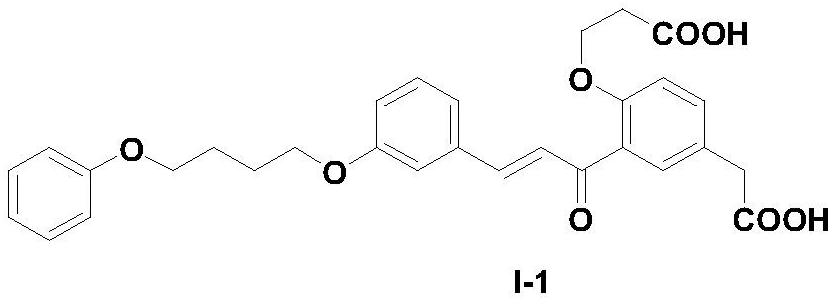
[0043]Example 1 3-(4-phenoxybutoxy)-2'-carboxyethoxy-5'-carboxymethylchalcone (I-1)
[0044]
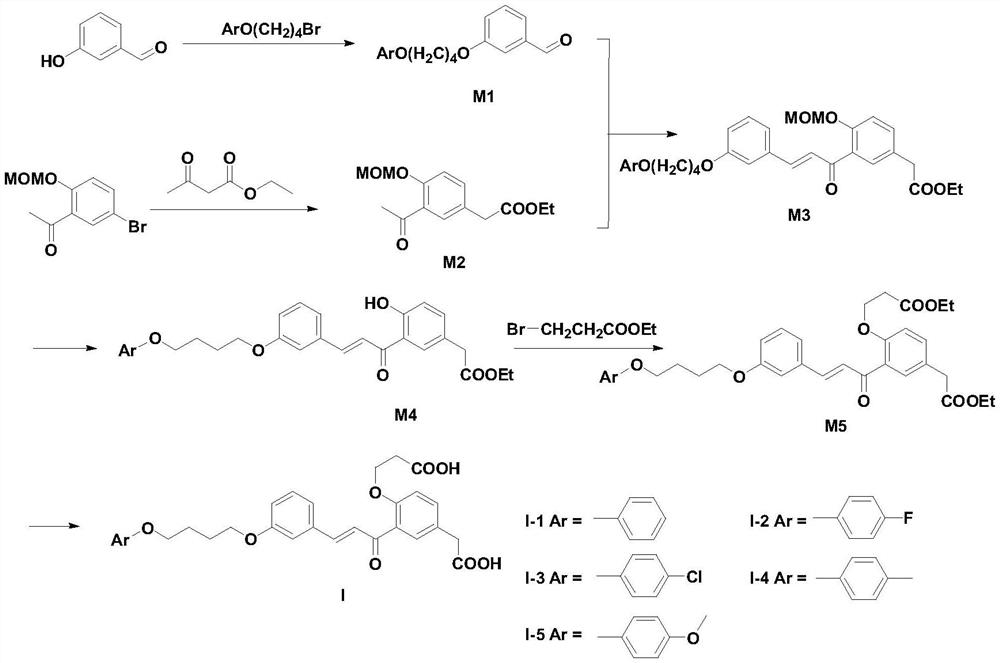
[0045] synthetic route:
[0046]
[0047] Synthesis of compound M1-1:
[0048] m-Hydroxybenzaldehyde (41.0mmol), 4-phenoxybutyl bromide (41.0mmol) and anhydrous potassium carbonate (82.0mmol) were dissolved in 200mL of acetone, heated to reflux for 8 hours, cooled, filtered to remove potassium carbonate, evaporated Acetone, and the residue was separated by silica gel column chromatography (petroleum ether:ethyl acetate=10:1, v / v) to obtain a pale yellow liquid (80%). MS:270[M] + .
[0049] Synthesis of Compound M2-1:
[0050] 2-Methoxymethoxy-5-bromoacetophenone (38.8 mmol), ethyl acetoacetate (59.1 mmol), potassium phosphate (116.4 mmol), palladium acetate (0.4 mmol) and tri-tert-butylphosphine ( 0.08mmol) into 20mL of toluene, heated to 150°C under the protection of nitrogen, reacted for 24 hours, cooled to room temperature, extracted with ethyl acetate, washed with satu...
Embodiment 2
[0055] Example 2 3-(4-p-fluorophenoxybutoxy)-2'-carboxyethoxy-5'-carboxymethylchalcone (I-2)
[0056]
[0057] 1-(4-bromobutoxy)-4-fluorobenzene was substituted for 4-bromobutoxybenzene in Example 1, and the synthesis method was referred to Example 1 to obtain compound I-2, MS:535[M-H] - . 1 H-NMR (400Hz, DMSO-d 6 ):δ7.91(s,1H),7.68(d,1H),7.51(d,1H),7.33(m,3H),7.27(d,1H),7.15(d,1H),7.04(m, 2H),6.91(d,1H),6.86(d,2H),4.35(t,2H),4.00(t,2H),3.96(t,2H),3.51(s,2H),2.78(t,2H ), 1.93(m,4H).
Embodiment 3
[0058] Example 3 3-(4-p-chlorophenoxybutoxy)-2'-carboxyethoxy-5'-carboxymethylchalcone (I-3)
[0059]
[0060] 1-(4-bromobutoxy)-4-chlorobenzene was substituted for 4-bromobutoxybenzene in Example 1, the synthetic method was referred to Example 1, and compound I-3 was synthesized, MS:551[M-H] - . 1 H-NMR (400Hz, DMSO-d 6 ):δ8.02(s,1H),7.87(d,1H),7.75(d,1H),7.62(d,1H),7.55(d,2H),7.46(d,1H),7.21(d, 1H),7.17(m,2H),7.04(m,3H),4.54(t,2H),4.28(t,2H),4.21(t,2H),3.72(s,2H),3.00(t,2H ), 2.03(m,4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com