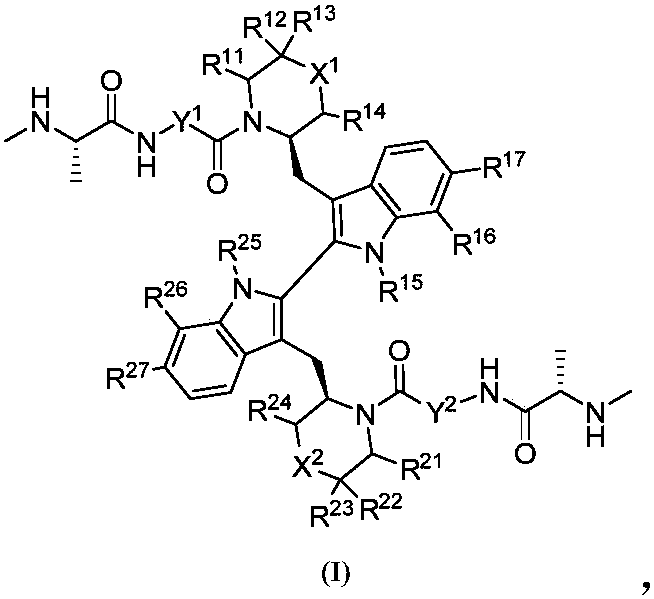
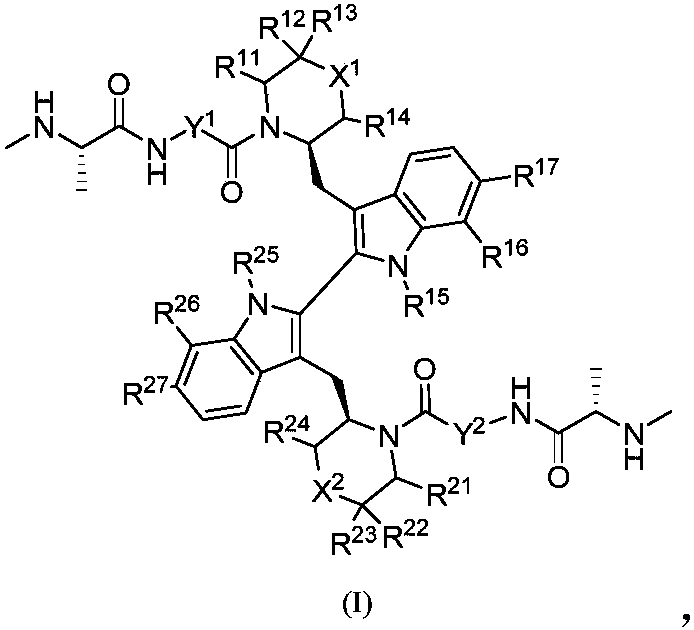
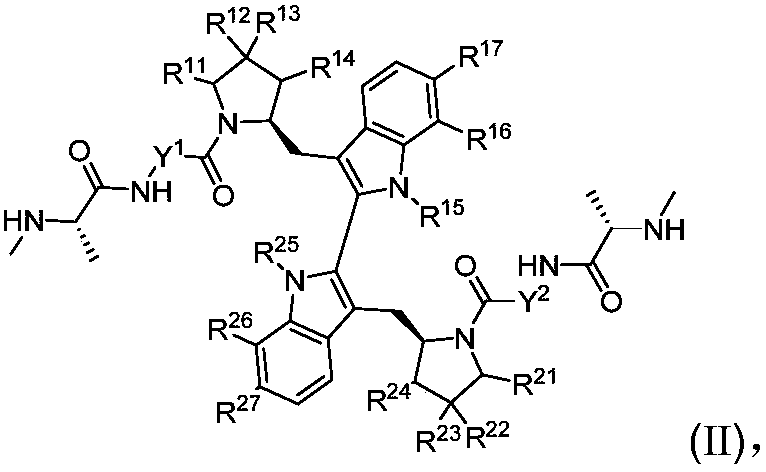
Compound as apoptosis protein inhibitor, and application thereof
A compound and solvate technology, applied to compounds as apoptotic protein inhibitors and their application fields, can solve the problems of difficult to achieve oral administration, toxic side effects, serious problems, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] Example 1: (2S,2'S)-N,N'-((2S,2'S)-((5R,5'R)-((6,6'-difluoro-1H,1'H-[2, 2'-biindole]-3,3'-bismethylene))bis(3,3-difluoropyrrolidine-5,1-diyl))bis(1-butyryl-1,2-di base)) bis(2-methylaminopropionamide)
[0117]
[0118] Step 1: Preparation of (2S,4R)-1-(benzyloxycarbonyl)-4-(tert-butyldimethylsilyloxy)pyrrolidine-2-carboxylic acid
[0119]
[0120] Dissolve L-N-Cbz-hydroxyproline (53.0 g, 200 mmol) in N,N-dimethylformamide (300 mL), then add imidazole (68.0 g, 1000 mmol) to its solution, followed by t-butyl Dimethylchlorosilane (60.0 g, 400 mmol) was stirred at room temperature for 16 h, water and ethyl acetate were added to the reaction solution, and the pH was adjusted to 3-4 with 6N hydrochloric acid. The organic phase was washed with water (500 mL×2), washed with saturated brine (300 mL), dried over anhydrous sodium sulfate, filtered with suction, concentrated and purified by column chromatography to obtain the title compound.
[0121] Step 2: Preparation of...
Embodiment 3
[0180] Example 3: (2S, 2'S)-N, N'-((2S, 2'S)-((3S, 3'S, 5R, 5'R)-((6,6'-difluoro-1H, 1'H -[2,2'-biindole]-3,3'-diyl)bis(methylene))bis(3-fluoropyrrolidine-5,1-diyl))bis(1-butyryl- 1,2-diyl)) dimethylamino) propionamide)
[0181]
[0182] Step 1: Preparation of (2R,4R)-4-acetoxy-2-(6-fluoro-1H-indol-3-yl)methylpyrrolidine-1-carboxylic acid benzyl ester
[0183]
[0184] (2R,4R)-2-(6-fluoro-1H-indol-3-yl)methyl-4-hydroxypyrrolidine-1-carboxylic acid benzyl ester (3.70g, 10.0mmol ), 4-dimethylaminopyridine (61.0mg, 0.500mmol) was dissolved in dichloromethane (50mL), then added acetic anhydride (2.00g, 20.0mmol), reacted overnight, then added methanol to quench (3mL), the reaction The system was successively washed with 10% aqueous sodium carbonate solution (30mL), 1N hydrochloric acid (30mL), and 10% aqueous sodium carbonate solution (30mL), and the organic phase was dried with anhydrous sodium sulfate, concentrated and separated by column chromatography (PE:EA =3:1-2:1)...
Embodiment 4
[0212] Example 4: (2S,2'S)-N,N'-((1S,1'S)-((3S,3'S,5R,5'R)-((6,6'-difluoro-1H,1'H -[2,2'-biindole]-3,3'-diyl)bis(methylene))bis(3-fluoropyrrolidine-5,1-diyl))bis(1-cyclohexyl- 2-oxoethane-2,1-diyl)(2-methylaminopropionamide)
[0213]
[0214] Step 1: ((1S,1'S)-((3S,3'S,5R,5'R)-((6,6'-difluoro-1H,1'H-[2,2'-biindole]- 3,3'-diyl)bis(methylene))bis(3-fluoropyrrolidine-5,1-diyl))bis(1-cyclohexyl-2-oxoethane-2,1-di base)) preparation of tert-butyl dicarbamate
[0215]
[0216] The preparation method is the same as ((2S,2'S)-((3S,3'S,5R,5'R)-((6,6'-difluoro-1H,1'H-[2,2' -biindole]-3,3'-diyl)bis(methylene))bis(3-fluoropyrrolidine-5,1-diyl))bis(1-butyryl-1,2-diyl) )) The preparation method of tert-butyl dicarbamate, the difference is that the raw material (S)-2-(tert-butoxycarbonylamino)butanoic acid is replaced by tert-butoxycarbonyl-L-cyclohexylglycine to obtain the title compound.
[0217] Step 2: (2S,2'S)-1,1'-((3S,3'S,5R,5'R)-((6,6'-difluoro-1H,1'H-[2,2'-link Indole]-3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com