Method for separating and preparing petunidin 3-galactoside chloride
A technology for petunin and galactoside, which is applied in the field of separation and preparation of petunin-3-O-galactoside, and can solve the problem of separation and preparation of petunin-3-O-galactoside that has not been found. Issues such as body research and reporting, to achieve the effect of large sample processing volume and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
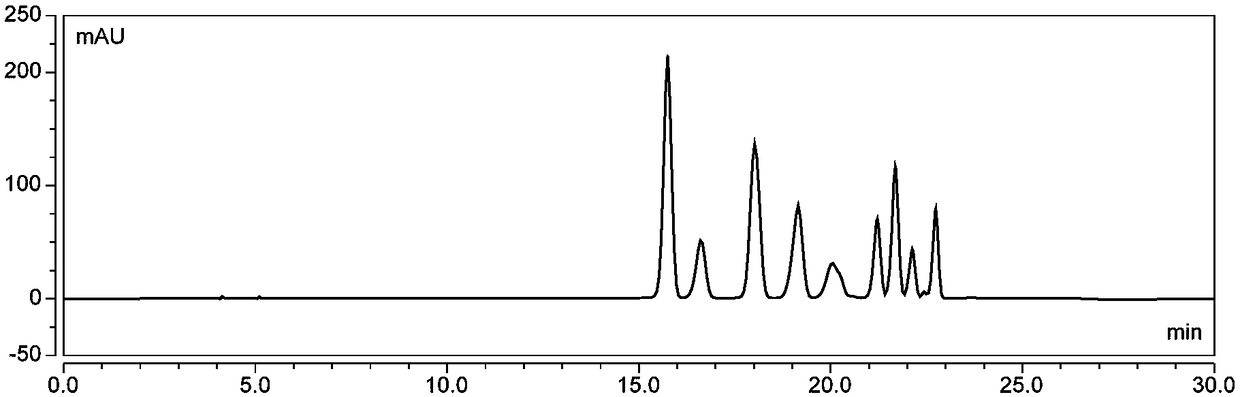
[0069] (1) Wash 1000g of fresh blueberries, add 70% ethanol aqueous solution containing 0.1% (v / v) hydrochloric acid (volume of ethanol and water) according to the ratio of solid to liquid 1:7 (w / v, g / mL) Ratio of 70:30) was fully mixed, ultrasonically extracted for 90 minutes, (45°C, protected from light), vacuum filtered, the filter residue was extracted twice according to the above conditions, the filtrate was combined, and ethanol was removed by vacuum rotary evaporation at 48°C to obtain crude anthocyanins extract;
[0070] (2) The AB-8 macroporous resin soaked in ethanol for 24h is packed into a chromatographic column, washed with deionized water until there is no alcohol smell, washed with 4% hydrochloric acid solution for 1h at a flow rate of 2BV / h, and then deionized Wash with ionized water until the effluent is neutral; wash with 4% sodium hydroxide solution at a flow rate of 2BV / h for 1 hour, and then wash with deionized water until neutral. Inject the crude anthoc...
Embodiment 2
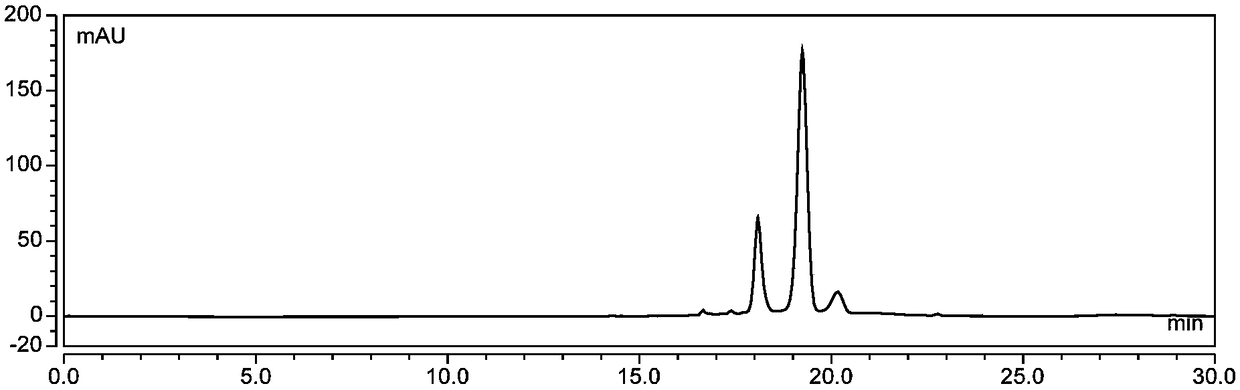
[0076] (1) Wash 3kg of fresh blueberries, add 80% ethanol aqueous solution containing 1.0% (v / v) hydrochloric acid according to the ratio of solid to liquid 1:9 (w / v) and mix thoroughly, ultrasonically extract for 120min, (45°C hereinafter, protected from light), filtered under reduced pressure, and the filter residue was repeatedly extracted 3 times according to the above conditions, and the filtrate was combined, and the ethanol was removed by vacuum rotary evaporation at 46°C to obtain a crude anthocyanin extract;
[0077] (2) The AB-8 macroporous resin soaked in ethanol for 24h is packed into a chromatographic column, washed with deionized water until there is no alcohol smell, washed with 4% hydrochloric acid solution for 1h at a flow rate of 2BV / h, and then deionized Wash with ionized water until the effluent is neutral; wash with 4% sodium hydroxide solution at a flow rate of 2BV / h for 1 hour, and then wash with deionized water until neutral. Inject the crude anthocyani...
Embodiment 3
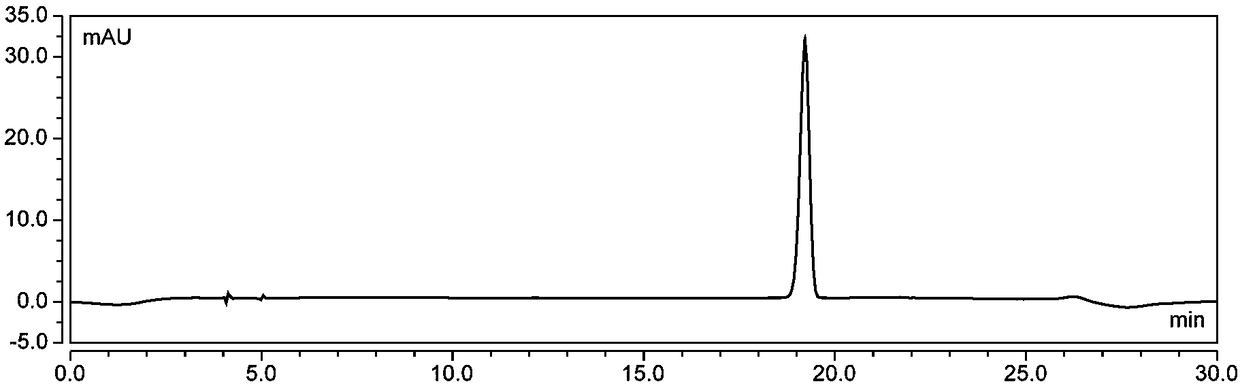
[0082] (1) Wash 5kg of fresh blueberries, add 90% ethanol aqueous solution containing 1.5% (v / v) hydrochloric acid according to the ratio of material to liquid (w / v) of 1:8 (w / v), mix thoroughly, and extract by ultrasonic for 200min, (45°C hereinafter, protected from light), filtered under reduced pressure, and the filter residue was repeatedly extracted 4 times according to the above conditions, the filtrates were combined, and the ethanol was removed by vacuum rotary evaporation at 47°C to obtain a crude anthocyanin extract;
[0083] (2) The AB-8 macroporous resin soaked in ethanol for 24h is packed into a chromatographic column, washed with deionized water until there is no alcohol smell, washed with 4% hydrochloric acid solution for 1h at a flow rate of 2BV / h, and then deionized Wash with ionized water until the effluent is neutral; wash with 4% sodium hydroxide solution at a flow rate of 2BV / h for 1 hour, and then wash with deionized water until neutral. Inject the crude an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com