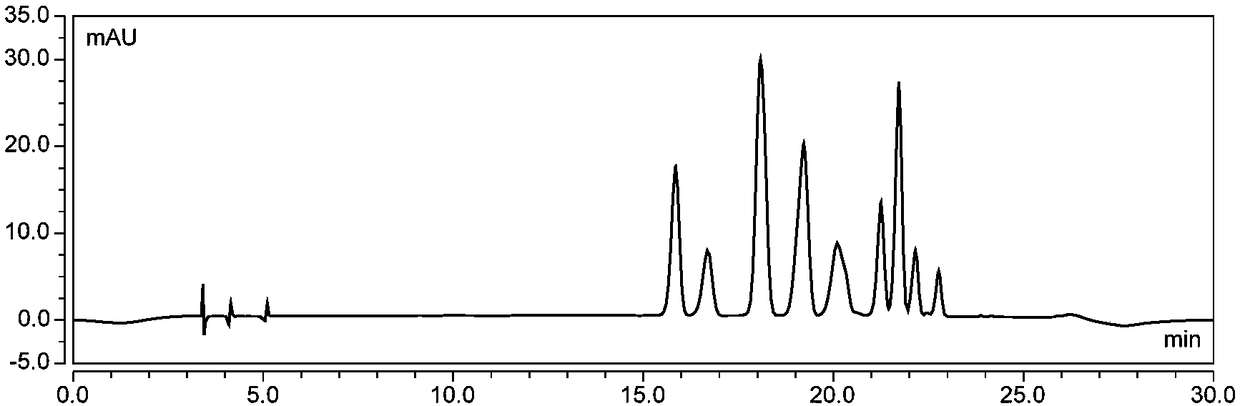
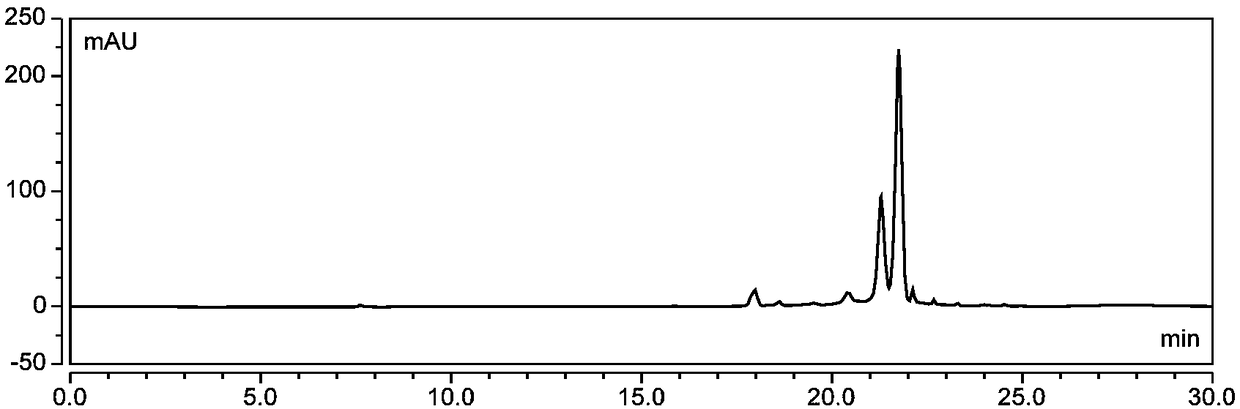
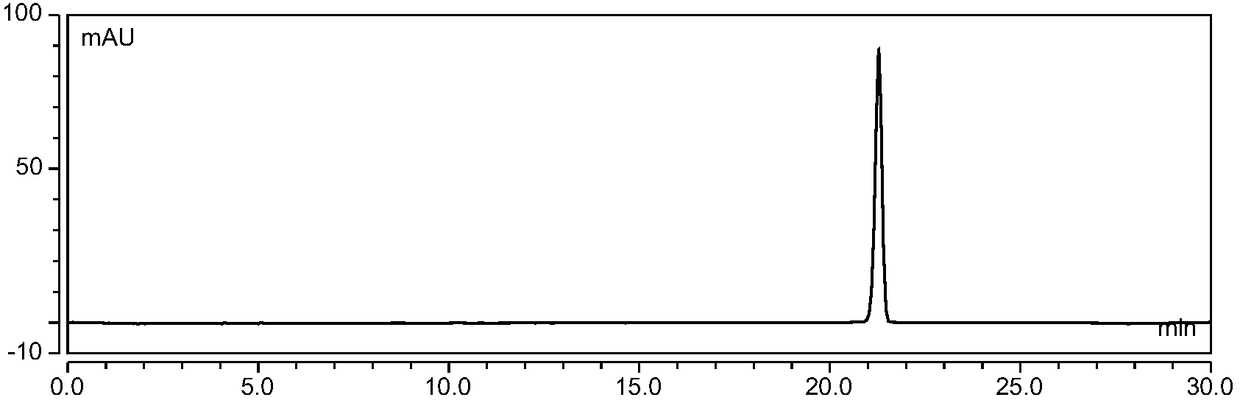
Method for separating and preparing petunidin 3-arabinoside chloride
A technology of arabinoside and petunia, which is applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve the undiscovered separation and preparation of petunin-3-O-arabinoside monomer research and reporting problems, to achieve the effect of large sample processing volume and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Wash 1kg of fresh blueberries, add 70% ethanol aqueous solution containing 0.1% (v / v) hydrochloric acid (the volume ratio of ethanol to water is 70 : 30) fully mixed, ultrasonically extracted for 90min, (control temperature below 45°C, protected from light), vacuum filtration after the end of ultrasonication, repeated extraction of the obtained filter residue once according to the above conditions, combined filtrate, and removed by vacuum rotary evaporation at 45°C Ethanol to obtain the crude extract of anthocyanins;
[0070] Soak the AB-8 macroporous resin in ethanol for 24 hours, put it into the chromatography column, wash it with pure water until there is no alcohol smell, wash it with 0.5M sodium hydroxide solution at a flow rate of 2BV / h for 1 hour, and then wash it with deionized water until the effluent is neutral; then use 0.5M hydrochloric acid solution to rinse for 1 hour at a flow rate of 2BV / h, and then rinse with deionized water until neutral. The crude an...
Embodiment 2
[0075] Wash 5kg of fresh blueberries, add 70% ethanol aqueous solution containing 0.5% (v / v) hydrochloric acid according to the ratio of material to liquid 1:7 (w / v) and mix thoroughly, ultrasonically extract for 150min, (control the temperature at 45°C hereinafter, protected from light), vacuum suction filtration after the end of ultrasonication, repeated extraction of the obtained filter residue once according to the above conditions, combined filtrates, and removed ethanol by vacuum rotary evaporation at 45°C to obtain crude anthocyanin extracts;
[0076] Soak the AB-8 macroporous resin in ethanol for 24 hours, put it into the chromatography column, wash it with pure water until there is no alcohol smell, wash it with 0.5M sodium hydroxide solution at a flow rate of 2BV / h for 1 hour, and then wash it with deionized water until the effluent is neutral; then use 0.5M hydrochloric acid solution to rinse for 1 hour at a flow rate of 2BV / h, and then rinse with deionized water unt...
Embodiment 3
[0080]Wash 10kg of fresh blueberries, add 60% ethanol aqueous solution containing 0.1% (v / v) hydrochloric acid according to the ratio of material to liquid (w / v) and mix thoroughly, and ultrasonically extract for 200min, (control the temperature at 45°C hereinafter, protected from light), vacuum suction filtration after the end of ultrasonication, repeated extraction of the obtained filter residue once according to the above conditions, combined filtrates, and removed ethanol by vacuum rotary evaporation at 45°C to obtain crude anthocyanin extracts;
[0081] Soak the AB-8 macroporous resin in ethanol for 24 hours, put it into the chromatography column, wash it with pure water until there is no alcohol smell, wash it with 0.5M sodium hydroxide solution at a flow rate of 2BV / h for 1 hour, and then wash it with deionized water until the effluent is neutral; then use 0.5M hydrochloric acid solution to rinse for 1 hour at a flow rate of 2BV / h, and then rinse with deionized water unt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com