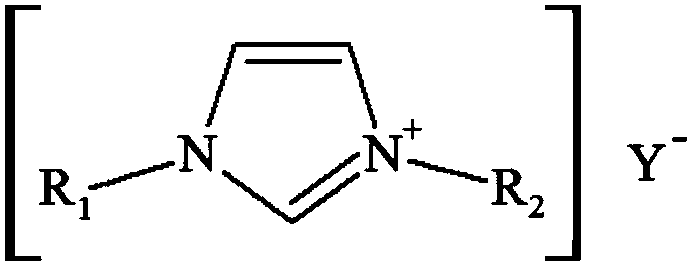
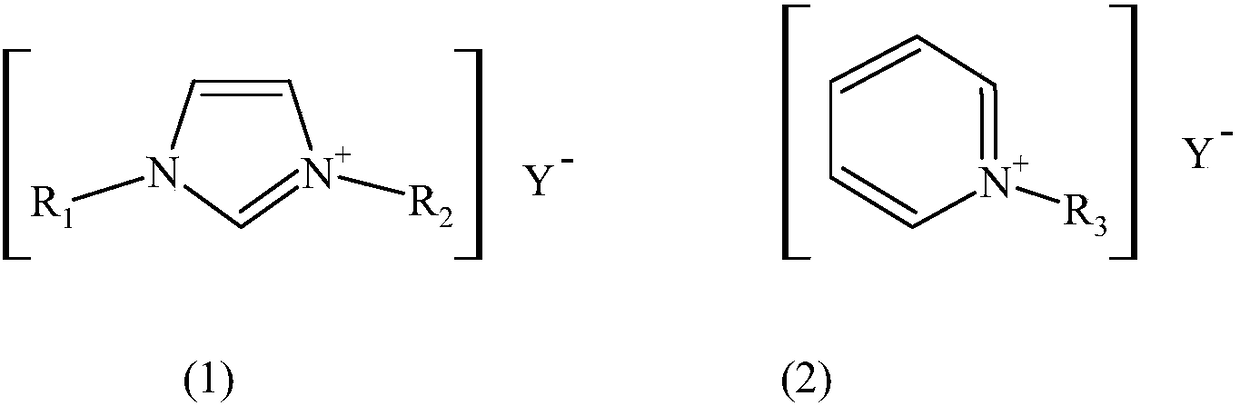Electrolyte and method for preparing adiponitrile by electrolytic acrylonitrile dimerization
A technology of acrylonitrile and electrolyte, applied in the direction of electrolysis process, electrolysis components, electrolysis organic production, etc., can solve the problems of corrosion electrolyte, poor stability, consumption, etc., and achieve the effect of reducing corrosion and stabilizing properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
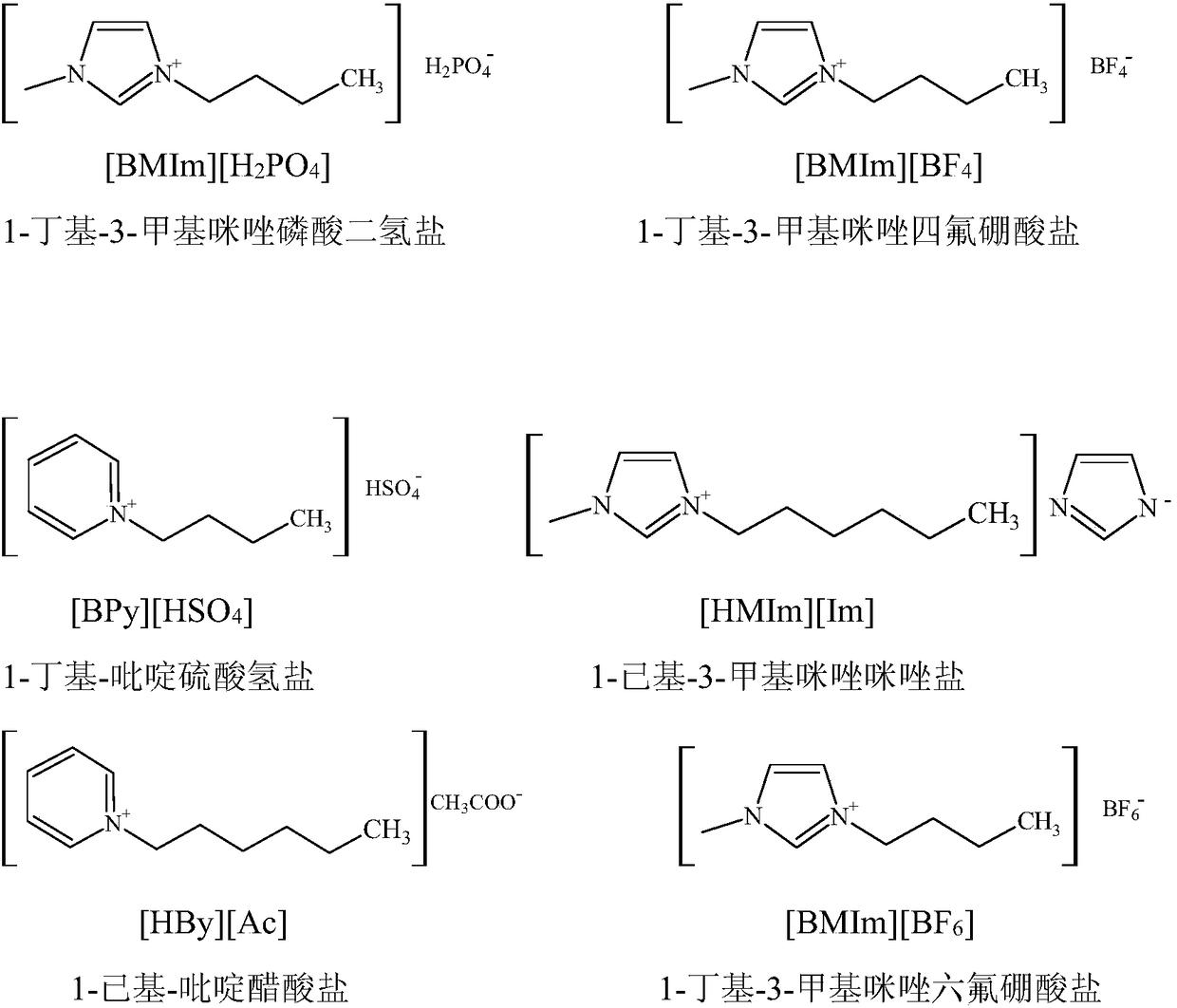
[0025] The electrolytic solution is prepared, and the electrolytic solution is composed of: 7wt% acrylonitrile, 2wt% ionic liquid [BMIm][H 2 PO 4 ], the EDTA sodium salt of 3wt%, the sodium hydrogen phosphate of 8wt%, all the other are water.
[0026] The electrolysis is carried out in a diaphragmless electrolytic cell, the anode material of the electrolytic cell is carbon steel, the cathode material is cadmium, and the effective size of each electrode plate is 50×3cm 2 .
[0027] The electrolysis conditions are: the temperature of the electrolyte is 40°C, and the linear velocity of the electrolyte between the electrode plates is 1m·s -1 , with a current density of 1500A m -2 .
[0028] After 24 hours of electrolysis, the yield of adiponitrile was 87.9%, and the current efficiency was 93.8%. Continuous electrolysis for 650h does not need to replenish ionic liquid.
Embodiment 2
[0030] Prepare the electrolyte, the composition of the electrolyte is: 2wt% acrylonitrile, 5wt% ionic liquid [BMIm][BF 4 ], 5wt% EDTA sodium salt, 15wt% potassium phosphate, and the rest are water.
[0031] The electrolysis is carried out in a diaphragmless electrolytic cell, the anode material of the electrolytic cell is carbon steel, the cathode material is cadmium, and the effective size of each electrode plate is 50×3cm 2 .
[0032] The electrolysis conditions are: the temperature of the electrolyte is 60°C, and the linear velocity of the electrolyte between the electrode plates is 1m·s -1 , with a current density of 4000A m -2 .
[0033] After 24 hours of electrolysis, the yield of adiponitrile was 85.1%, and the current efficiency was 91.2%. Continuous electrolysis for 650h does not need to replenish ionic liquid.
Embodiment 3
[0035]Prepare the electrolyte, the composition of the electrolyte is: 5wt% acrylonitrile, 5wt% ionic liquid [BPy][HSO 4 ], 0.1wt% potassium salt of EDTA, 5wt% sodium dihydrogen phosphate, and the rest are water.
[0036] The electrolysis is carried out in a diaphragmless electrolytic cell, the anode material of the electrolytic cell is graphite, the cathode material is lead, and the effective size of each electrode plate is 50×3cm 2 .
[0037] The electrolysis conditions are: the temperature of the electrolyte is 20°C, and the linear velocity of the electrolyte between the electrode plates is 1.2m s -1 , with a current density of 500A m -2 .
[0038] After 48 hours of electrolysis, the yield of adiponitrile was 86.3%, and the current efficiency was 92.5%. Continuous electrolysis for 650h does not need to replenish ionic liquid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| velocity | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com