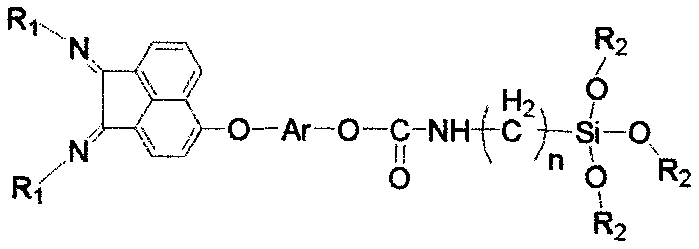
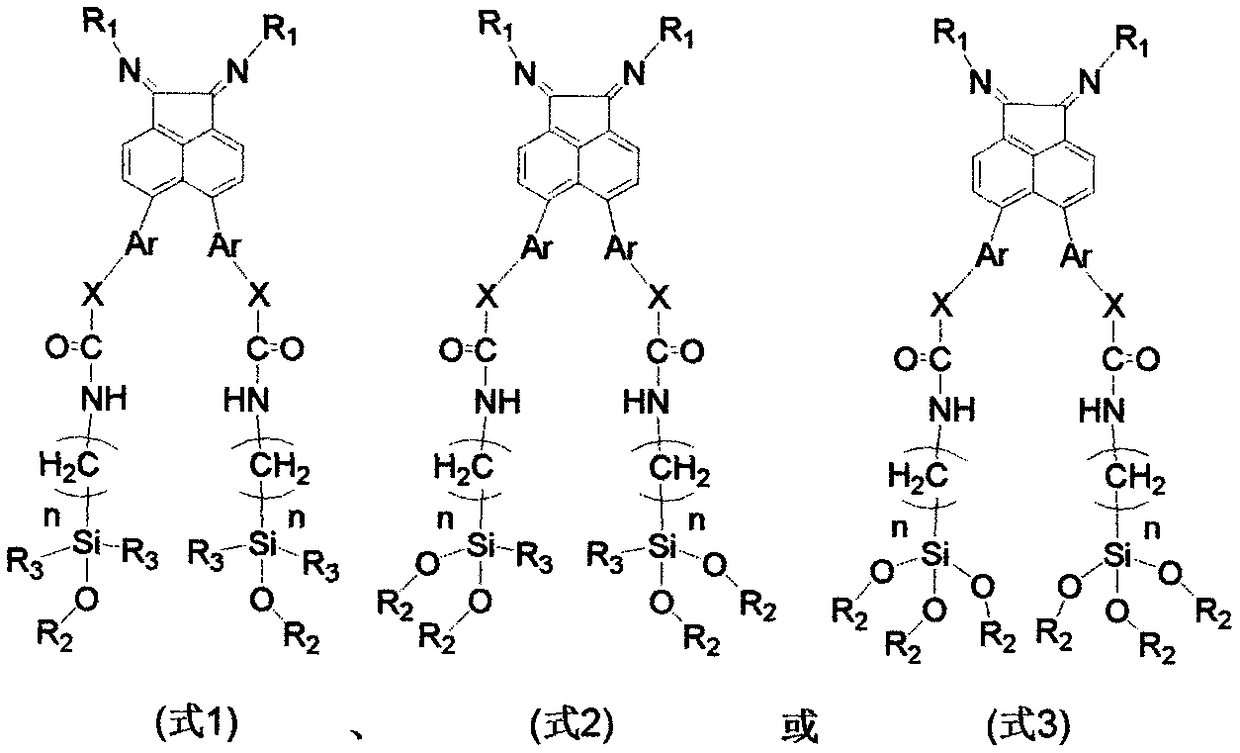
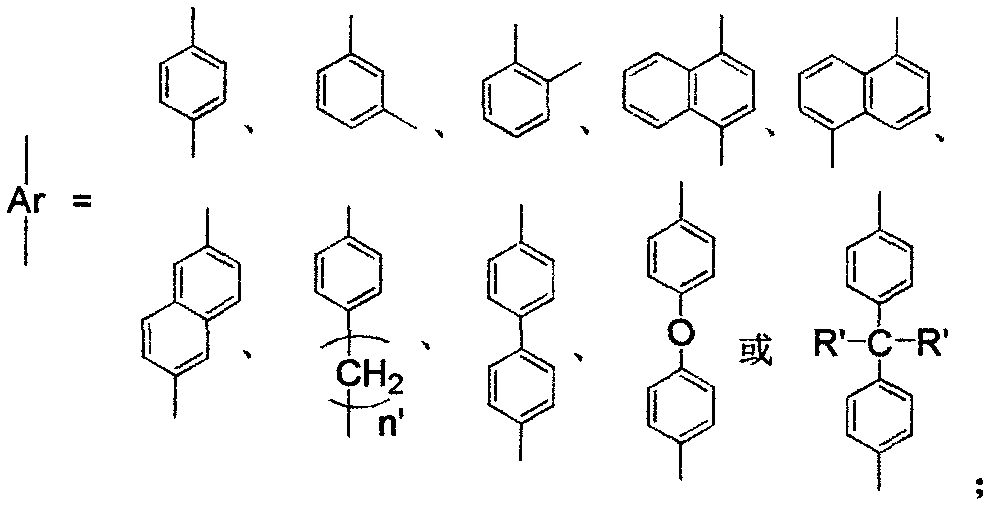
Two-alkoxyl-silicon-containing alpha-diimine compound and application of load type metal complex thereof in olefin polymerization
A technology of diimine compound and bisalkoxy silicon is applied in the application field of α-diimine compound and its supported metal complex in olefin polymerization, which can solve the problem of not very firm ligand loading, and achieves the The effect of strong chemical bond force, reduced influence and large adjustable range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Synthesis of load f1
[0033] Its preparation route is as follows:
[0034]
[0035] Synthesis of 5,6-dibromoacenaphthenequinone bis(2,4-dimethyl-6-benzhydryl)phenylimine a1:
[0036] Add compound 5,6-dibromoacenaphthenequinone (2.72g, 8mmol), 2,4-dimethyl-6-benzhydrylaniline (5.46g, 19mmol) and p-toluene to the reaction flask of 250mL Sulfonic acid (3.27g, 19mmol) and 150mL of anhydrous toluene, the mixture was heated to reflux, and the water formed during the reaction was removed. The reaction was tracked by chromatography until the reaction of the raw materials was complete, and a clear wine red solution was obtained. After the reaction solution was concentrated, the reaction mixture was separated by column chromatography to obtain 6.05g red solid a1 (i.e. the structure of compound A, wherein R 1 =[2-Benzhydryl-4,6-dimethyl]phenyl), the yield was 86%. 1 H NMR (400MHz, CDCl 3 ): δ7.69(d, J=7.8Hz, 2H), 7.28(d, J=8.1Hz, 2H), 7.25-7.17(m, 8H), 7.11(s, 2H), 6.91(d...
Embodiment 2
[0044] Synthesis of load g1
[0045] Its preparation route is as follows:
[0046]
[0047] SiO 2 / MgCl 2 Preparation of composite carrier:
[0048] Add the solvent n-heptane 50mL and 1g of anhydrous MgCl in sequence in a five-neck flask with mechanical stirring under the protection of argon 2 , and a certain amount of n-butanol was added at 35°C (the molar ratio of n-butanol to magnesium chloride was 4.0:1), and then the temperature was programmed to rise to 90°C for 3h to obtain a colorless transparent solution. Then the solution was cooled to 60°C, and a certain amount of SiO was added 2 (MgCl 2 and SiO 2 Mass ratio is equal to 0.05), and after stirring at this temperature for 2h, stop, and obtain SiO through suction filtration and drying 2 / MgCl 2 Composite carrier.
[0049] 5,6-bis[4-(3-(monoethyldipropoxysilyl)propylcarbamatemethyl)phenyl]acenaphthenequinonebis(2,4-dimethyl-6-benzhydryl) Benzene imine d1 (i.e. the structure of D compound, where R 1 =[2-benz...
Embodiment 3
[0051] Synthesis of cargo h1
[0052] Its preparation route is as follows:
[0053]
[0054] 5,6-bis[4-(3-dimethyldimethoxysilyl)propylcarbamatemethyl)phenyl]acenaphthenequinonebis(2,4-dimethyl-6-benzhydryl)benzene Imine e1 (that is, the structure of the E compound, where R 1 =[2-benzhydryl-4,6-dimethyl]phenyl, Ar=benzyl, X=O, R 2 = methyl, R 3 =methyl) was synthesized in the same steps as that of c1 in Example 1. The specific preparation process of the load h1 is the same as that of the load f1 in Example 1, wherein diatomaceous earth is used instead of SiO in Example 1 2 . Elemental analysis of load h1: C, 19.8%; N, 1.1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight average molecule | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com