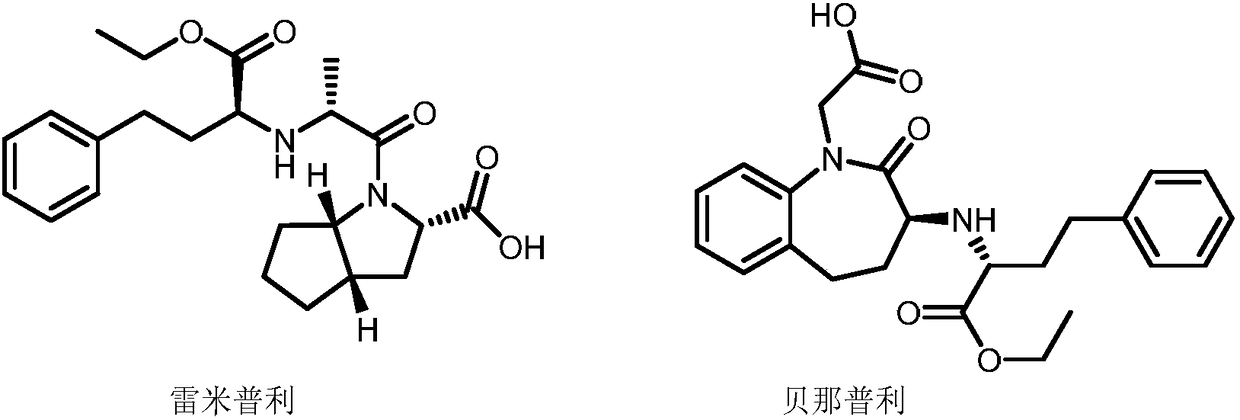
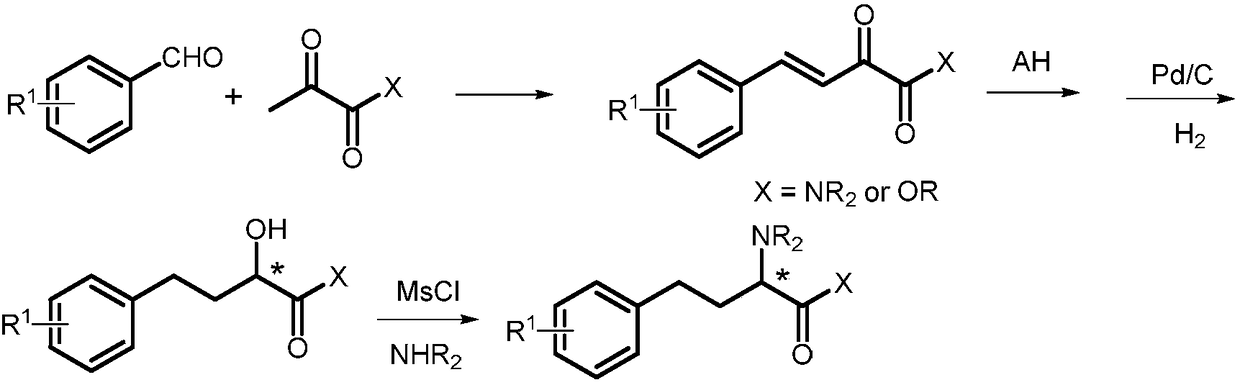
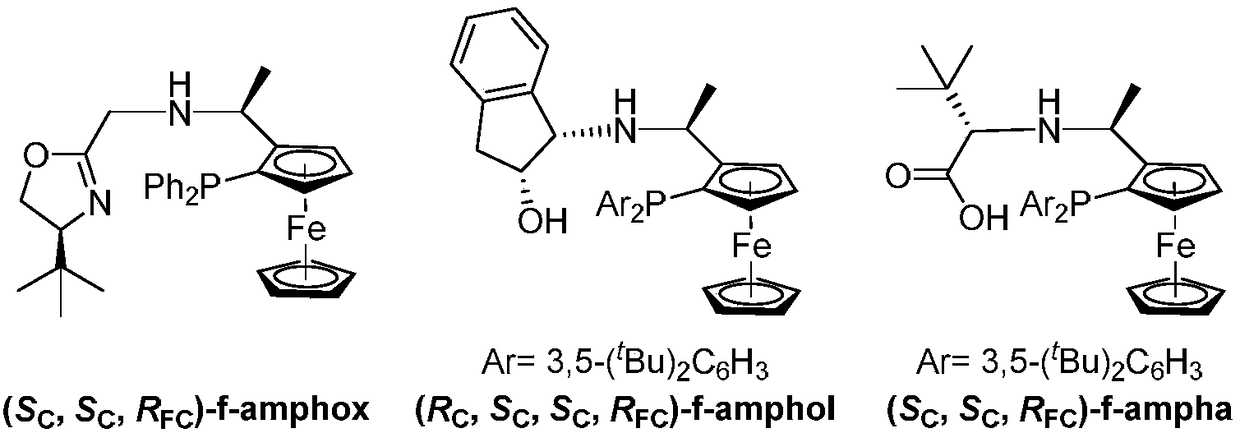
Asymmetric hydrogenation method of alpha-ketone amide compound
A compound and asymmetric technology, applied in the field of asymmetric catalysis, can solve the problems of reproducibility and economic efficiency, and achieve good industrial application prospects, high conversion rate and selectivity, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Preparation of 2a by hydrogenation of 1a
[0041]
[0042] In an argon-filled glove box, add the metal precursor [Ir(COD)Cl] to a 10 mL vial 2 (4.0mg, 5.9×10 -3 mmol), ligand L1 (7.3mg, 13.1×10 -3 mmol) and anhydrous i PrOH (3 mL). The mixture was stirred at 25 °C for 2 h to obtain an orange-red solution. The resulting solution (50 μL, c=4×10 -3 mmol / mL) and t BuOK at i The solution in PrOH (40 μL, c=0.05mmol / mL) was transferred to the i PrOH (2mL) solution in a 5mL vial. Transfer the vial to an autoclave and fill with 50 atm of H 2 , and stirred at room temperature for 12h. Hydrogen gas was slowly released in a well-ventilated fume hood. After the solution was concentrated, the metal complexes were removed through a silica gel column to obtain product 2a. According to nuclear magnetic analysis, the conversion rate was 99%, and through HPLC analysis, the measured ee value was 92%.
[0043] 1 H NMR (400MHz, CDCl 3 )δ7.39–7.34(m,2H),7.34–7.19(m,8H),6.75(s...
Embodiment 2
[0046] Preparation of 2a by hydrogenation of 1a
[0047] In an argon-filled glove box, add the metal precursor [Ir(COD)Cl] to a 10 mL vial 2 (4.0mg, 5.9×10 -3 mmol), ligand L2 (7.3mg, 13.1×10 -3 mmol) and anhydrous i PrOH (3 mL). The mixture was stirred at 25 °C for 2 h to obtain an orange-red solution. The resulting solution (50 μL, c=4×10 -3 mmol / mL) and t BuOK at i The solution in PrOH (40 μL, c=0.05mmol / mL) was transferred to the i PrOH (2mL) solution in a 5mL vial. Transfer the vial to an autoclave and fill with 50 atm of H 2 , and stirred at room temperature for 12h. Hydrogen gas was slowly released in a well-ventilated fume hood. After the solution was concentrated, the metal complexes were removed through a silica gel column to obtain product 2a. According to nuclear magnetic analysis, the conversion rate was 30%, and through HPLC analysis, the measured ee value was 81%.
[0048]
Embodiment 3
[0050] Preparation of 2a by hydrogenation of 1a
[0051] In an argon-filled glove box, add the metal precursor [Ir(COD)Cl] to a 10 mL vial 2 (4.0mg, 5.9×10 -3 mmol), ligand L3 (7.3mg, 13.1×10 -3 mmol) and anhydrous i PrOH (3 mL). The mixture was stirred at 25 °C for 2 h to obtain an orange-red solution. The resulting solution (50 μL, c=4×10 -3 mmol / mL) and t BuOK at i The solution in PrOH (40 μL, c=0.05mmol / mL) was transferred to the i PrOH (2mL) solution in a 5mL vial. Transfer the vial to an autoclave and fill with 50 atm of H 2 , and stirred at room temperature for 12h. Hydrogen gas was slowly released in a well-ventilated fume hood. After the solution was concentrated, the metal complexes were removed through a silica gel column to obtain product 2a. According to nuclear magnetic analysis, the conversion rate was 36%, and through HPLC analysis, the measured ee value was 84%.
[0052]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com