Method for catalyzing selective hydrogenolysis of lignin by zirconium phosphate loaded nickel-based material
A nickel-based catalyst and lignin technology, applied in chemical instruments and methods, catalyst activation/preparation, physical/chemical process catalysts, etc., can solve problems such as harsh reaction conditions, high production costs, and expensive raw materials, and achieve equipment The effect of low requirements, low price and simple process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
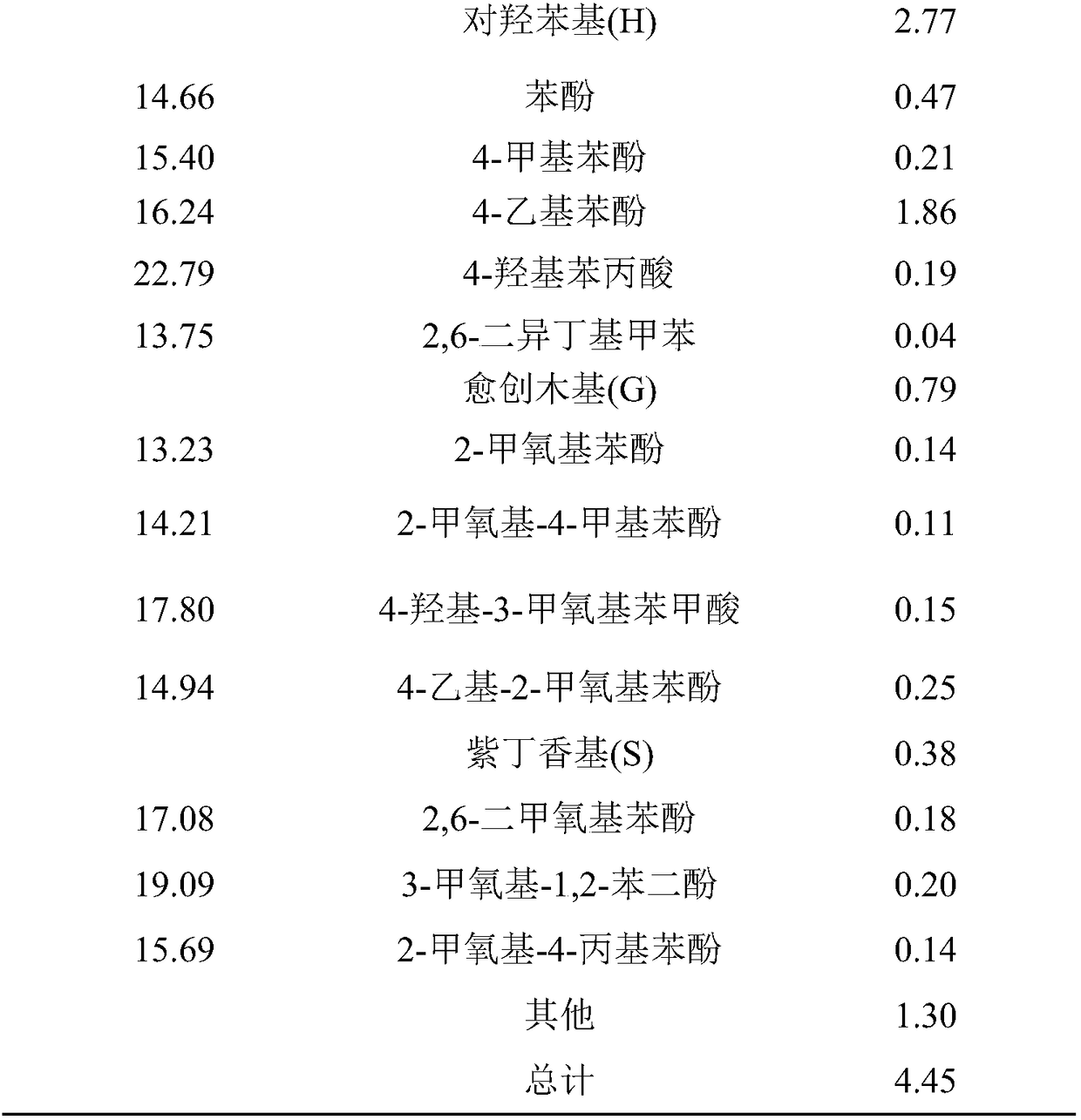
Image
Examples
Embodiment 1
[0022] Embodiment 1: the preparation of catalyst 20wt%Ni / ZrP-2
[0023] (1) Weighing 23.02g of ZrOCl with a purity of 98% 2 ·8H 2 O solid, fully dissolved with 70ml deionized water for use, similarly, weigh 18.77g of NH with a purity of 98.5% 4 h 2 PO 4 The solid was dissolved in 140 ml deionized water. Then pour the two into a 250ml round-bottomed flask in turn, while fully stirring. Filter until a white emulsion is formed, and carefully wash the solid part with deionized water several times until no obvious precipitation is formed. Take the solid part and place it in an oven at 120°C for 12h, and then calcinate it in a muffle furnace at 550°C for 4h, and the obtained white powder is the catalyst carrier ZrP-2.
[0024] (2) Weigh 2.225g of Ni(NO 3 ) 2 ·6H 2 O was dissolved in 0.55 ml of deionized water, and after the dissolution was complete, 2 g of the carrier ZrP-2 prepared in step (1) was added. After immersion for 12 h, it was dried in a 120 °C drying oven for 2...
Embodiment 2
[0026] Embodiment 2: the preparation of 15wt%Ni / ZrP-1
[0027] The difference of this embodiment, such as embodiment 1, is:
[0028] (1) Catalyst carrier raw material ZrOCl 2 ·8H 2 O and NH 4 h 2 PO 4 The amount ratio of substances is 1:1;
[0029] (2) The loading amount of the catalyst active component Ni is 15wt%.
Embodiment 3
[0030] Embodiment 3: the preparation of 15wt%Ni / ZrP-2.5
[0031] The difference of this embodiment, such as embodiment 1, is:
[0032] (1) Catalyst carrier raw material ZrOCl 2 ·8H 2 O and NH 4 h 2 PO 4 The molar ratio of substances is 2.5:1;
[0033] (2) The loading amount of the catalyst active component Ni is 15wt%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com