Method for preparing diketopyrrolopyrrole organic materials with terminals substituted by dye units
A technology of diketopyrrolopyrrole and organic materials, applied in the field of synthesis of organic conjugated small molecules, which can solve problems such as difficult large-scale production, hazards of chloroform solvents, skin irritation, etc., achieve low toxicity, reduce energy consumption, and facilitate operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
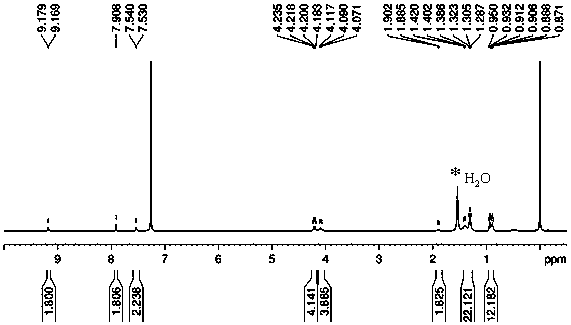
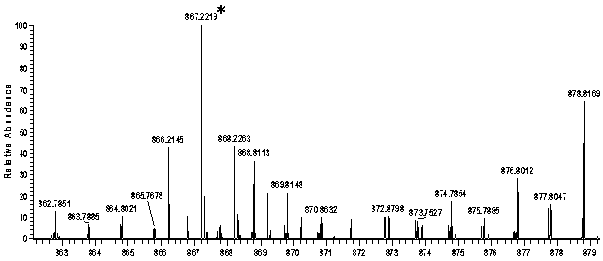
Image
Examples
Embodiment 1
[0017] Add compound 1 (0.2 mmol, 116 mg) and 3-ethylrhodanine (1.0 mmol, 161 mg) in a molar ratio of 1:5 in a 50 mL round bottom flask, add 20 mL of dichloromethane and 0.02 mmol of tris Ethylamine was kept at room temperature for 10 hours. After the reaction, the system was washed three times with water, and the organic phase was washed with anhydrous MgSO 4 After drying, the solvent was removed, and the crude product was recrystallized from a solution of dichloromethane and methanol at a volume ratio of 20:1 to obtain a purple solid powder product with a yield of 65%.
Embodiment 2
[0019] Add compound 1 (0.2 mmol, 116 mg) and 3-ethylrhodanine (2.0 mmol, 322 mg) in a molar ratio of 1:10 in a 50 mL round bottom flask, add 20 mL of dichloromethane and 0.02 mmol of tris Ethylamine was kept at room temperature for 10 hours. After the reaction, the system was washed three times with water, and the organic phase was washed with anhydrous MgSO 4 After drying, the solvent was removed, and the crude product was recrystallized from a solution of dichloromethane and methanol at a volume ratio of 20:1 to obtain a purple solid powder product with a yield of 87%.
Embodiment 3
[0021] Add compound 1 (0.2 mmol, 116 mg) and 3-ethylrhodanine (4.0 mmol, 644 mg) in a molar ratio of 1:20 in a 50 mL round bottom flask, add 20 mL of dichloromethane and 0.02 mmol of tris Ethylamine was kept at room temperature for 10 hours. After the reaction, the system was washed three times with water, and the organic phase was washed with anhydrous MgSO 4 After drying, the solvent was removed, and the crude product was recrystallized from a solution of dichloromethane and methanol at a volume ratio of 20:1 to obtain a purple solid powder product with a yield of 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com