Synthesis process of dimethylamine borane
A dimethylamine borane, synthesis process technology, applied in the preparation of amino compounds from amines, inorganic chemistry, metal hydrides, etc., can solve the problems of sacrificing reaction efficiency and reaction safety, and achieve the possibility of avoiding safety risks, Efficiency improvement and size reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
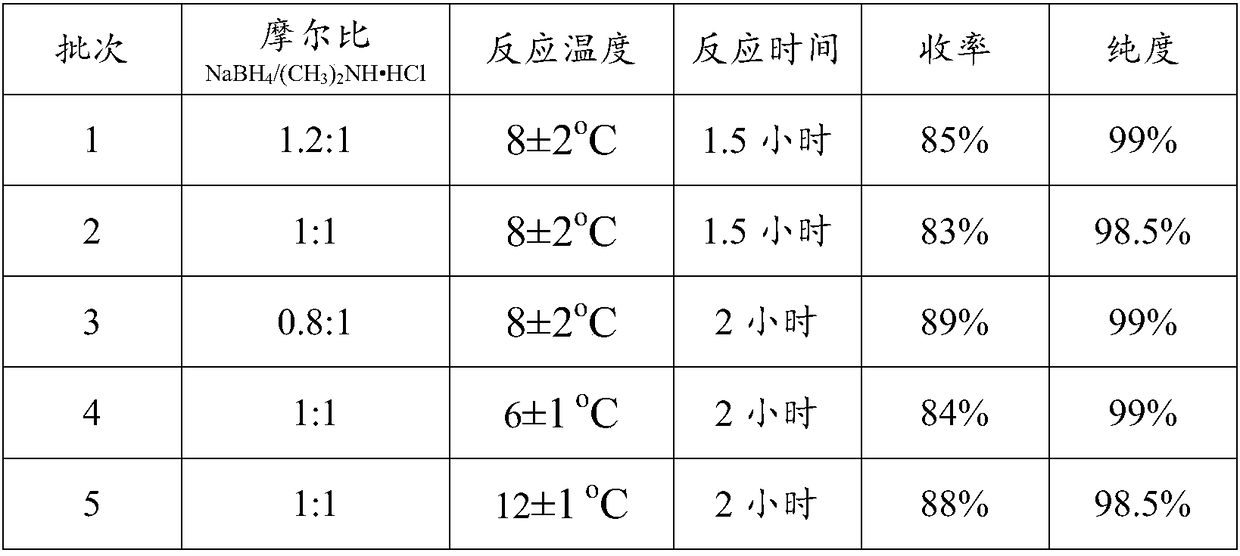
[0032] Continuously fill the 1000mL reaction flask with nitrogen to maintain the flow rate of nitrogen, add 30.7g (0.82mol) sodium borohydride and 100mL tetrahydrofuran into the reaction flask, control the temperature at 8±2°C, and add the dihydrofuran in 5 batches under vigorous stirring. Methylamine hydrochloride 55.3g (0.68mol), fully reacted for 1 to 2 hours; the obtained reaction solution was filtered, the filtrate was directly subjected to vacuum distillation, and then saturated sodium hydroxide solution was added to the concentrated solution obtained by distillation, and after stirring, Stand still, remove the lower alkali aqueous solution, and directly cool and crystallize the upper layer solution in a solvent-free state to obtain 40.8 g of dimethylamine borane as a solid, with a yield of 85% and a purity of 99%, as shown in batch 1 in the following table;
[0033] According to the same synthesis method as batch 1, the sodium borohydride / dimethylamine hydrochloride feed...
Embodiment 2
[0045] The solution of the present invention-industrial grade
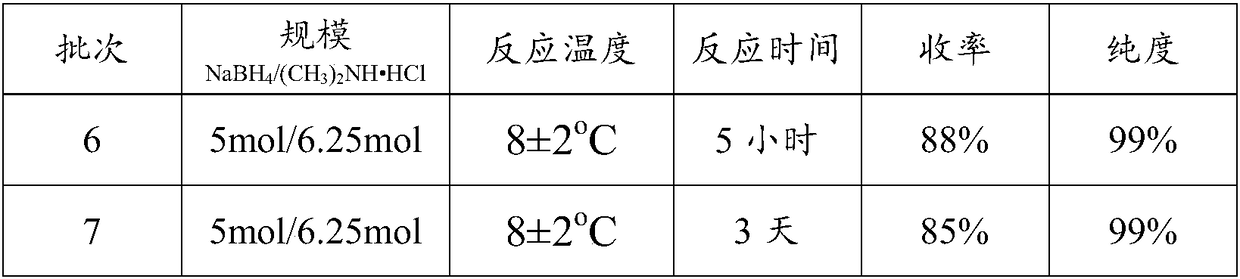
[0046]Continuously fill the 5000mL reaction flask with nitrogen to maintain the flow rate of nitrogen, add 189.15g (5mol) sodium borohydride and 700mL tetrahydrofuran into the reaction flask, control the temperature at 8±2°C, and add dimethylformamide in 10 batches under vigorous stirring Amine hydrochloride 509.62g (6.25mol), fully reacted 5 hours; The obtained reaction solution was filtered, and the filtrate was directly subjected to vacuum distillation, then in the concentrated solution obtained by distillation, a saturated sodium hydroxide solution was added, stirred, and then separated Remove the lower alkali aqueous solution, and directly cool and crystallize the upper layer solution in a solvent-free state to obtain 259.25 g of dimethylamine borane as a solid, with a yield of 88% and a purity of 99%. See Batch 6 in the following table;
[0047] Control scheme - industrial grade
[0048] Add 189.15g (5mol) ...
Embodiment 3
[0052] The solution of the present invention-industrial grade
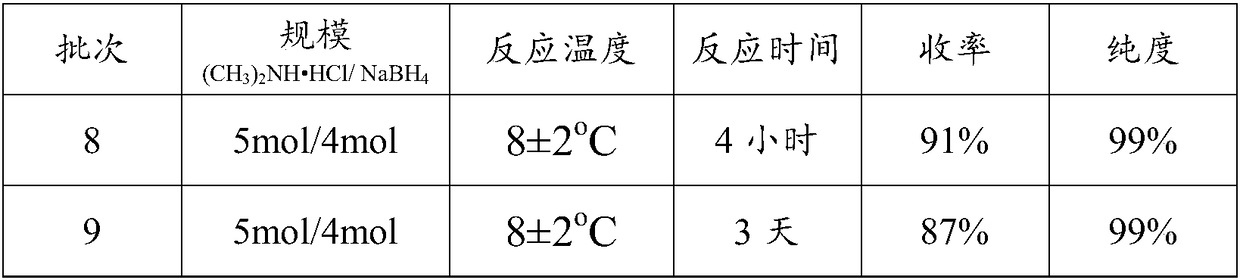
[0053] Fill the 5000mL reaction flask with nitrogen continuously to maintain the nitrogen flow rate, add 407.7g (5mol) dimethylamine hydrochloride and 1000mL tetrahydrofuran into the reaction flask, control the temperature at 8±2°C, and divide into 5 batches under vigorous stirring Add 151.32 g (4 mol) of sodium borohydride and react fully for 4 hours; filter the obtained reaction solution, and directly carry out vacuum distillation on the filtrate, then add saturated sodium hydroxide solution to the concentrated solution obtained by distillation, stand still after stirring, and separate The lower layer of alkali aqueous solution, the upper layer solution was directly cooled and crystallized in a solvent-free state, and 214.46 g of dimethylamine borane solid was obtained, with a yield of 91% and a purity of 99%. See batch 8 in the following table;
[0054] Control scheme - industrial grade
[0055] Add 407.7g (5m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com