Compound as well as preparation method and application thereof
A compound and complex technology, applied in the field of compounds and their preparation, can solve the problems of unfavorable drug purity of canagliflozin, unknown impurity structure, control of production process, etc., and achieves easy control of process operation, low cost, and improved quality Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
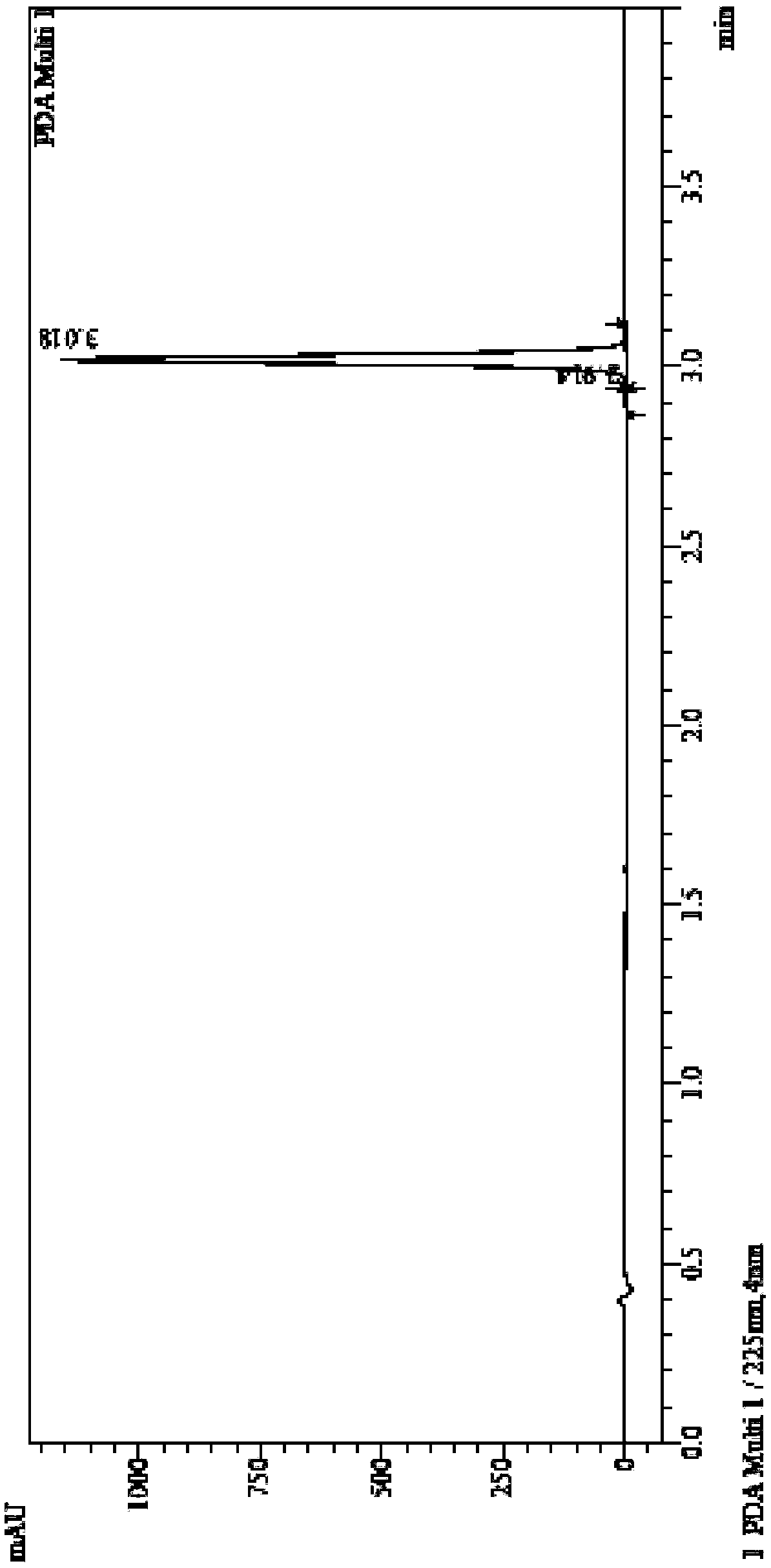
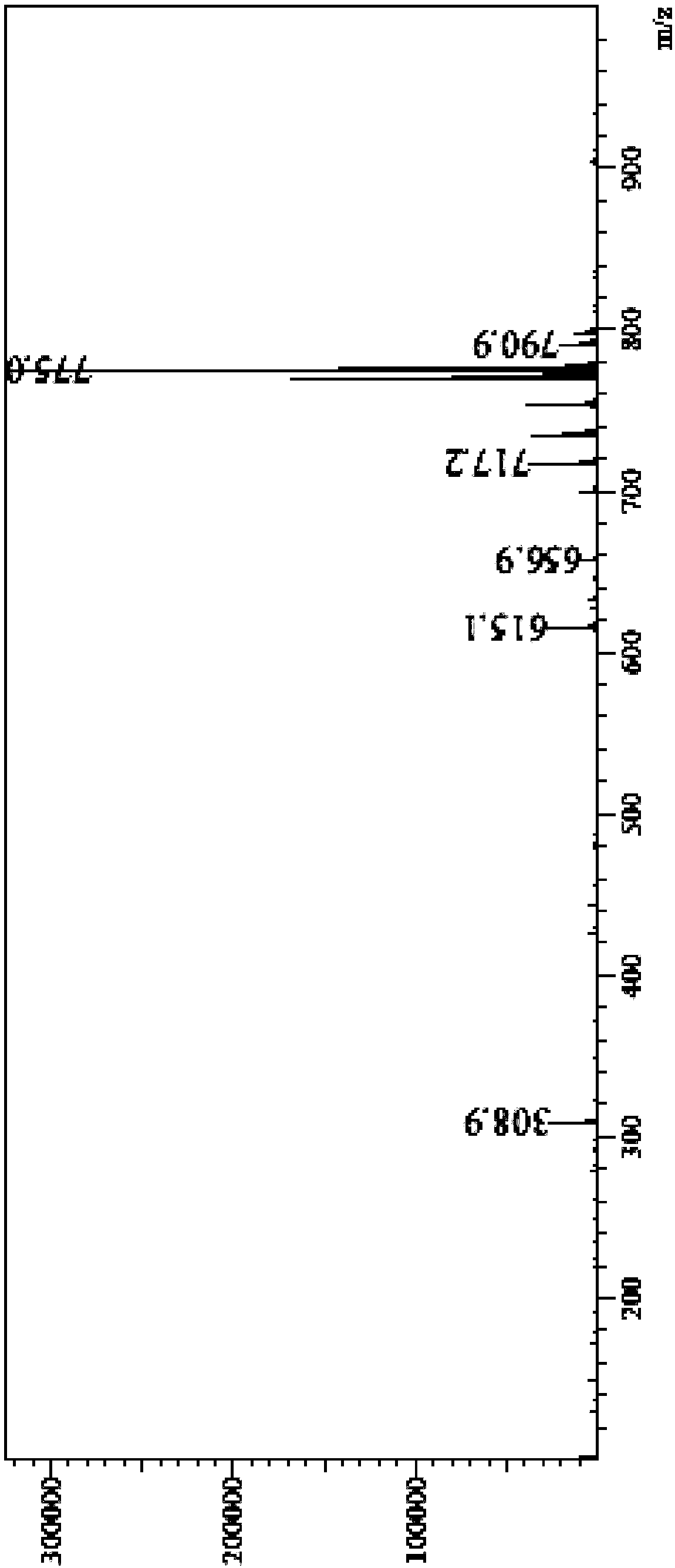
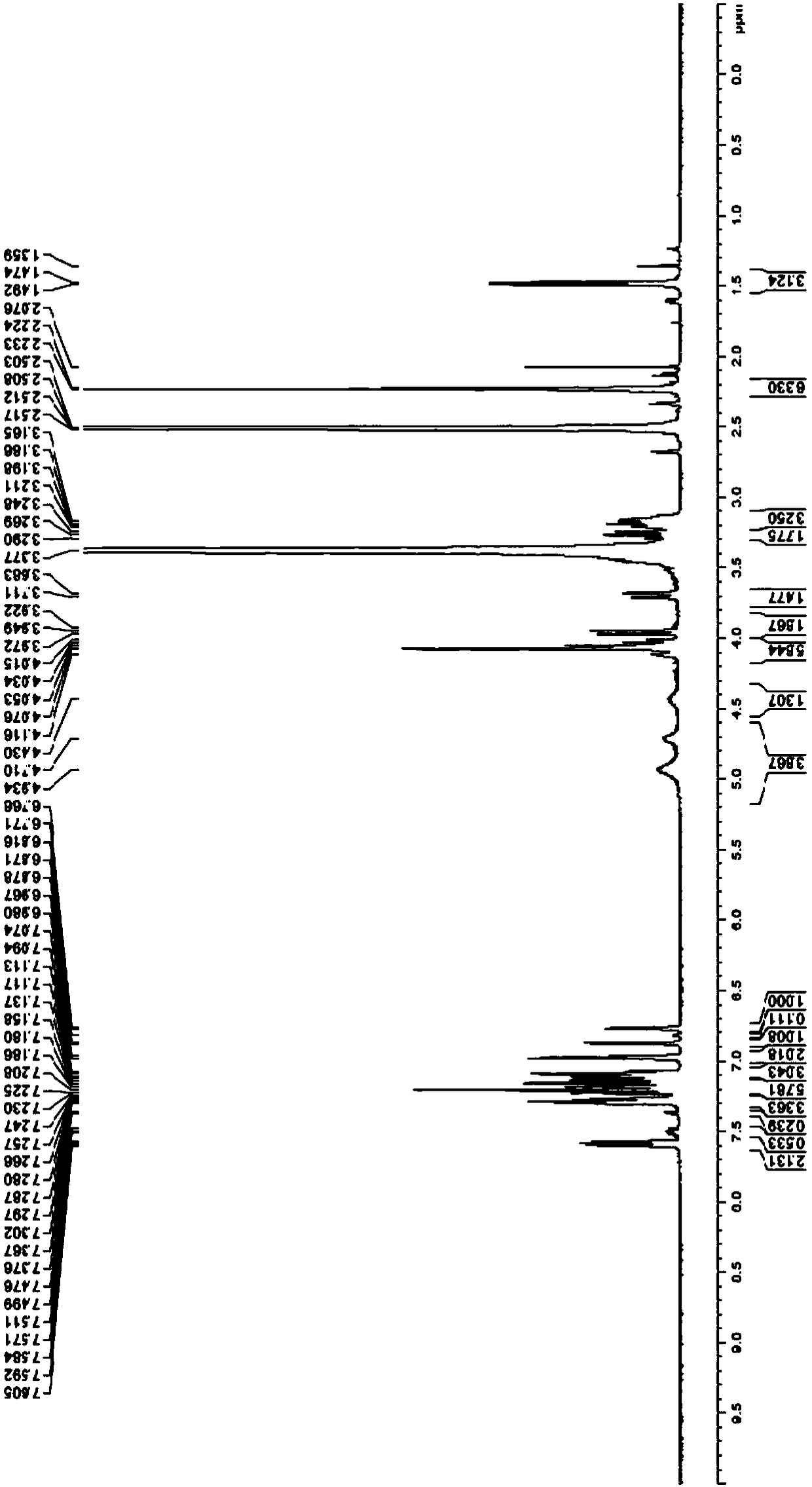
Image
Examples
Embodiment 1
[0065]
[0066] S1. Add 20g of D-gluconolactone into a 100ml dry three-necked flask, add 70g of acetic anhydride, add 30g of cation exchange resin at room temperature, and keep it at 40-50°C for 2-3 hours after the addition is completed. TLC monitors that the reaction solution is basically No gluconolactone. After the reaction was completed, the cation exchange resin was filtered out, and the filtrate was concentrated under reduced pressure at 70-80°C until there was no distillate, and about 41 g of compound 1 was obtained as a brownish-yellow oily viscous liquid, with a yield of about 105%.
[0067] S2. In a 100ml dry three-neck flask, add 30ml of toluene, 13g, cooled down to 0-10°C under the protection of nitrogen, 30ml of tetrahydrofuran solution of sec-butylmagnesium chloride and lithium chloride was added under stirring, and kept at -10-0°C for 1-2 hours to obtain the prepared Grignard reagent.
[0068] S3. Add 20g of compound 1 and 20ml of tetrahydrofuran into anothe...
Embodiment 2
[0072]
[0073] S1. Add 300g of D-gluconolactone into a 1000ml dry three-necked flask, add 800g of acetic anhydride, add 450g of cation exchange resin at room temperature, and keep it at 40-50°C for 6-8 hours after the addition is completed. TLC monitors that the reaction solution is basically No gluconolactone. After the reaction was completed, the cation exchange resin was filtered out, and the filtrate was concentrated under reduced pressure at 70-80°C until there was no distillate, and about 642 g of brown oily viscous liquid Compound 1 was obtained, with a yield of about 110%.
[0074] S2. In a 100ml dry three-neck flask, add 30ml of toluene, 15g, under the protection of nitrogen, lower the temperature to 0-10°C, add 30ml of tetrahydrofuran solution of sec-butylmagnesium chloride and lithium chloride under stirring, and keep it at -10-0°C for 1-2 hours to obtain the prepared Grignard reagent.
[0075] S3~S5 are the same as embodiment 1.
experiment example
[0077] The following is a method for qualitative and quantitative detection of the canagliflozin impurity formula I prepared in Example 2 of the present invention. The present invention finds that the prepared canagliflozin impurity formula I can make the quality detection of the finished product of canagliflozin more accurate and controllable, and in the finished product of canagliflozin produced by the original research process route, the impurity also remains in the finished product If the structure of the impurity formula I of canagliflozin is not determined, the related substances in the canagliflozin bulk drug cannot be effectively controlled.
[0078] The method for detecting the finished product of canagliflozin by adopting the impurity formula I of canagliflozin prepared by the present invention below.
[0079] Related substances: get about 25 mg of canagliflozin, dissolve and quantitatively dilute with acetonitrile-water (80:20) to make the test solution containing a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com