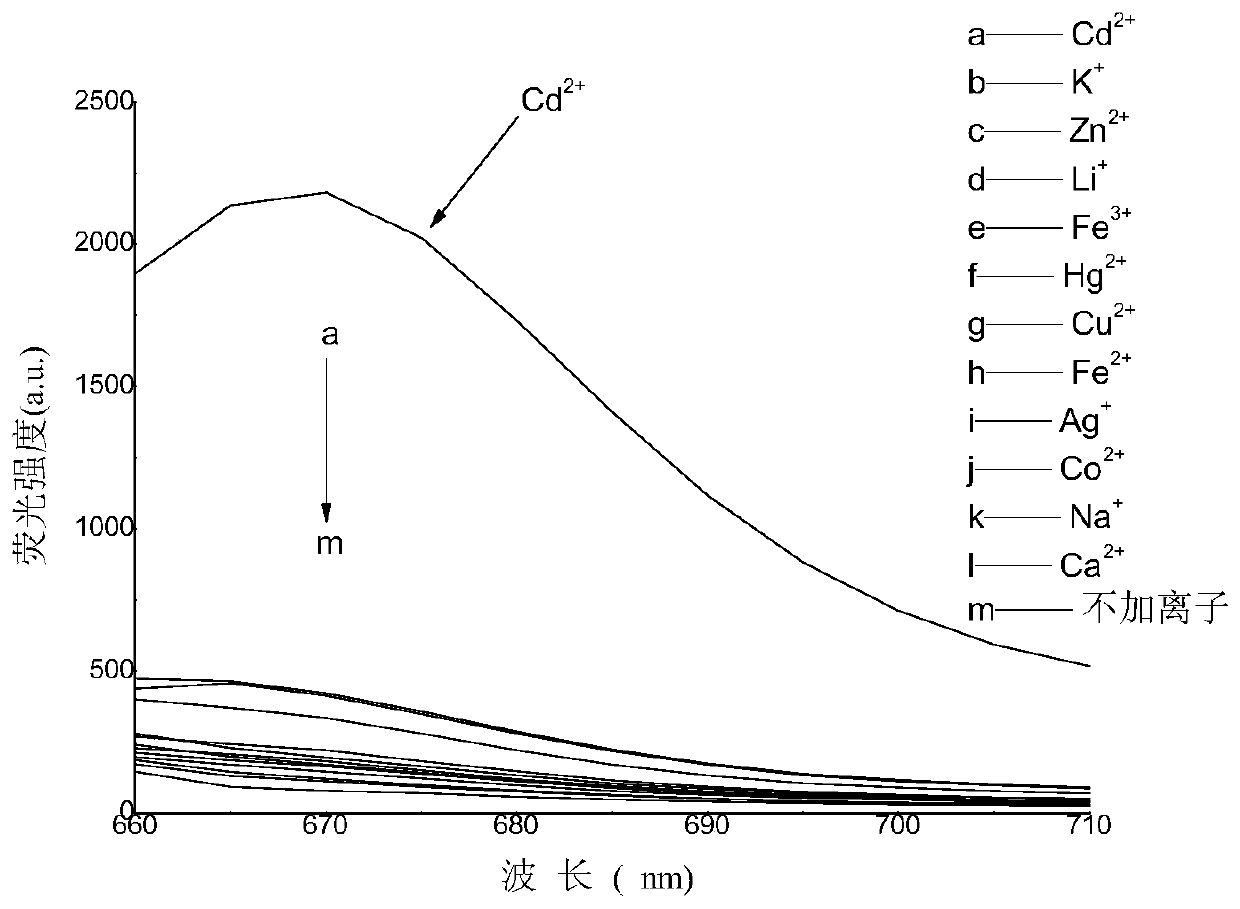
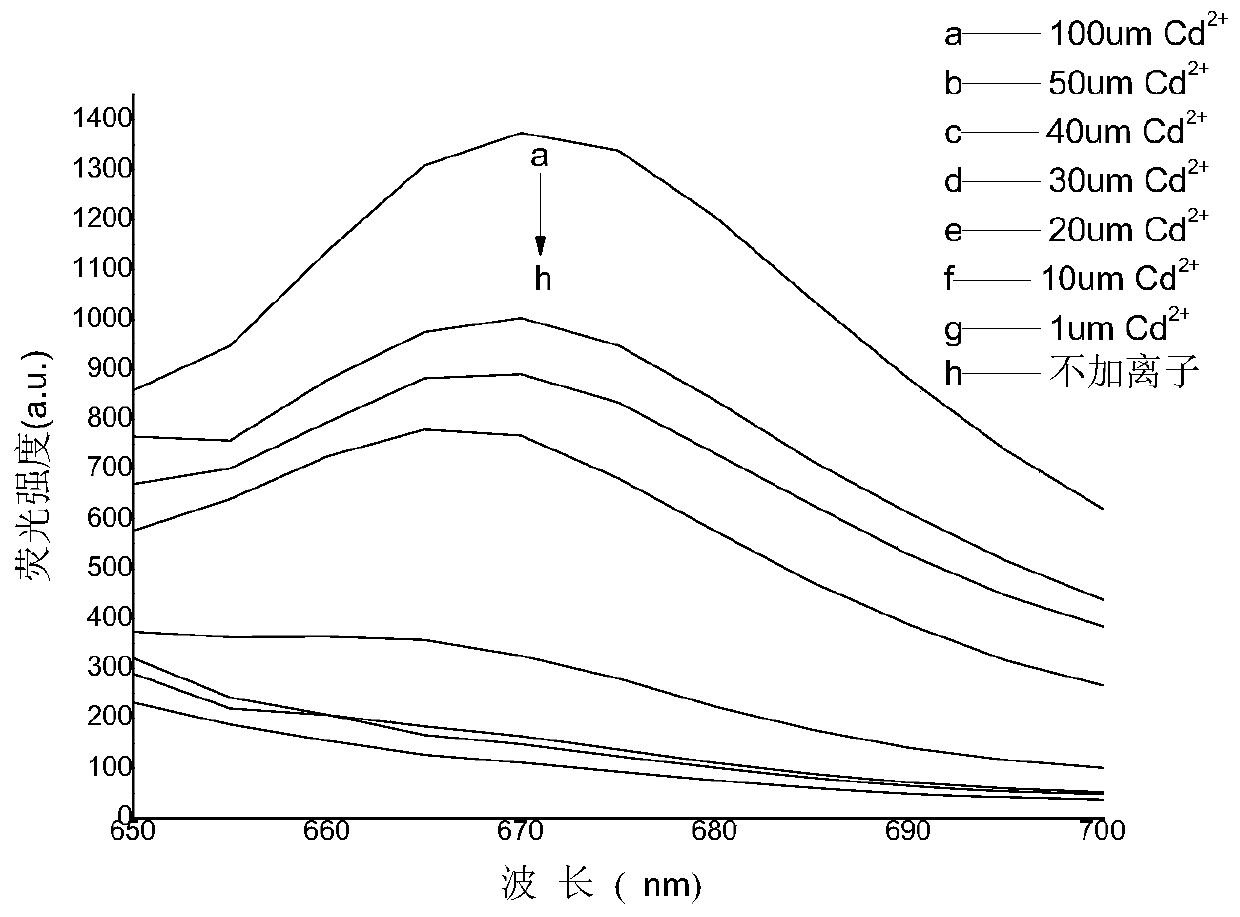
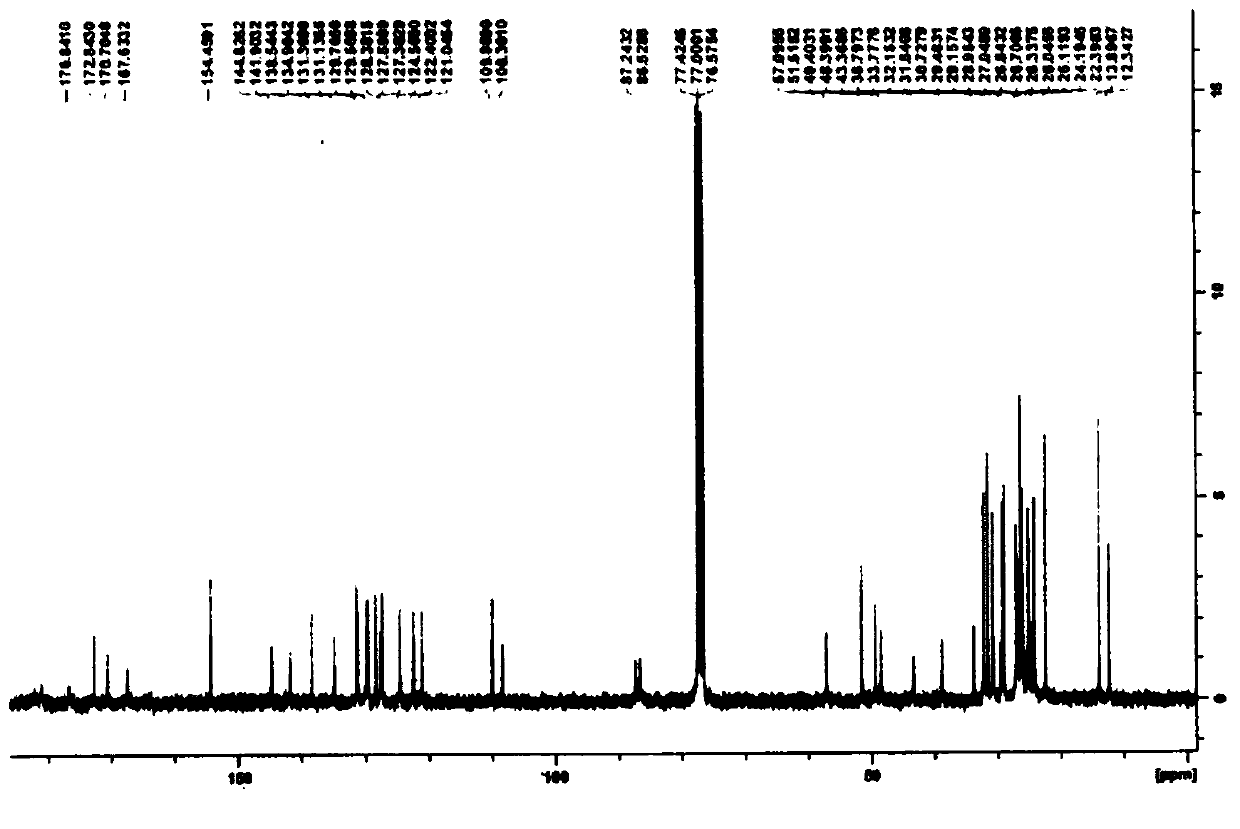
A guanine-based squaraine probe and its preparation method and application
A technology of guanine and probe, which is applied in the field of guanine-based squarylium probe and its preparation, can solve the problem of poor selectivity of ion-selective electrode method, low sensitivity of oscillographic polarography, and low sensitivity of refractory elements. Advanced problems, to achieve the effect of easy control of reaction conditions, high sensitivity and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (1) Dissolve Intermediate 1 (0.1903g, 0.6mmol) and Intermediate 2 (0.1806g, 0.6mmol) in a mixed solution of 15mL n-butanol and 15mL toluene, reflux at 110°C for 12 hours under nitrogen, and remove As a solvent, use a developing solvent of dichloromethane and methanol (volume ratio 10:1) to pass through a silica gel column to obtain 0.19 g of intermediate 3 as a blue solid.
[0024] (2) Intermediate 3 (0.0630g, 0.1mmol) obtained in step (1) and 2-amino-1,7-dihydro-6H-purin-6-one (0.0333g, 0.2mmol) were dissolved in 4 -Dimethylaminopyridine (0.0252g, 0.2mmol) was stirred in a dichloromethane solution at room temperature for 1 hour, then dicyclohexylcarbodiimide (0.0315g, 0.15mmol) was added to continue the reaction for 27 hours, and the solvent was removed under reduced pressure to obtain The blue solid was separated and purified by thin-layer chromatography (volume ratio of developer: methylene chloride: methanol = 25:1) to obtain 0.0606 g of the final product.
[0025]...
Embodiment 2
[0030] (1) Dissolve Intermediate 1 (0.3806g, 1.2mmol) and Intermediate 2 (0.3612g, 1.2mmol) in a mixed solution of 30mL n-butanol and 30mL toluene, reflux at 110°C for 12 hours under nitrogen, and remove The solvent was passed through a silica gel column with a developing solvent of dichloromethane and methanol (volume ratio = 10:1), and spin-dried to obtain 0.38 g of intermediate 3 as a blue solid.
[0031] (2) Compound 3 (0.1260g, 0.2mmol) obtained in step (1) and 2-amino-1,7-dihydro-6H-purin-6-one (0.0666g, 0.4mmol) were dissolved in 4- A dichloromethane solution of dimethylaminopyridine (0.0504g, 0.4mmol) was stirred at room temperature for 1 hour, and then dicyclohexylcarbodiimide (0.0630g, 0.3mmol) was added to continue the reaction for 27 hours. Color solid, thin-layer chromatography (volume ratio of developer: dichloromethane: methanol = 25:1) separation and purification to obtain the final product 0.1209g.
Embodiment 3
[0033] (1) Dissolve Intermediate 1 (0.3806g, 1.2mmol) and Intermediate 2 (0.3612g, 1.2mmol) in a mixed solution of 30mL n-butanol and 30mL toluene, reflux at 120°C for 13 hours under nitrogen, and remove The solvent was passed through a silica gel column with a developing solvent of dichloromethane and methanol (volume ratio = 10:1), and spin-dried to obtain 0.41 g of intermediate 3 as a blue solid.
[0034] (2) Compound 3 (0.2520g, 0.4mmol) obtained in step (1) and 2-amino-1,7-dihydro-6H-purin-6-one (0.1332g, 0.8mmol) were dissolved in 4- A dichloromethane solution of dimethylaminopyridine (0.1008g, 0.8mmol) was stirred at room temperature for 1 hour, and then dicyclohexylcarbodiimide (0.1260g, 0.6mmol) was added to continue the reaction for 30 hours. A color solid was separated and purified by thin-layer chromatography (volume ratio of developer: methylene chloride: methanol = 25:1) to obtain 0.2365 g of the final product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com