Ferric phosphate as well as preparation method and application thereof
A technology of iron phosphate and iron source, applied in chemical instruments and methods, phosphorus compounds, structural parts, etc., can solve the problems of affecting the electrochemical performance of lithium iron phosphate batteries, low density of iron phosphate, and increasing production costs, etc., to achieve inhibition Effects of self-discharge, avoidance of by-product salt, and extended life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
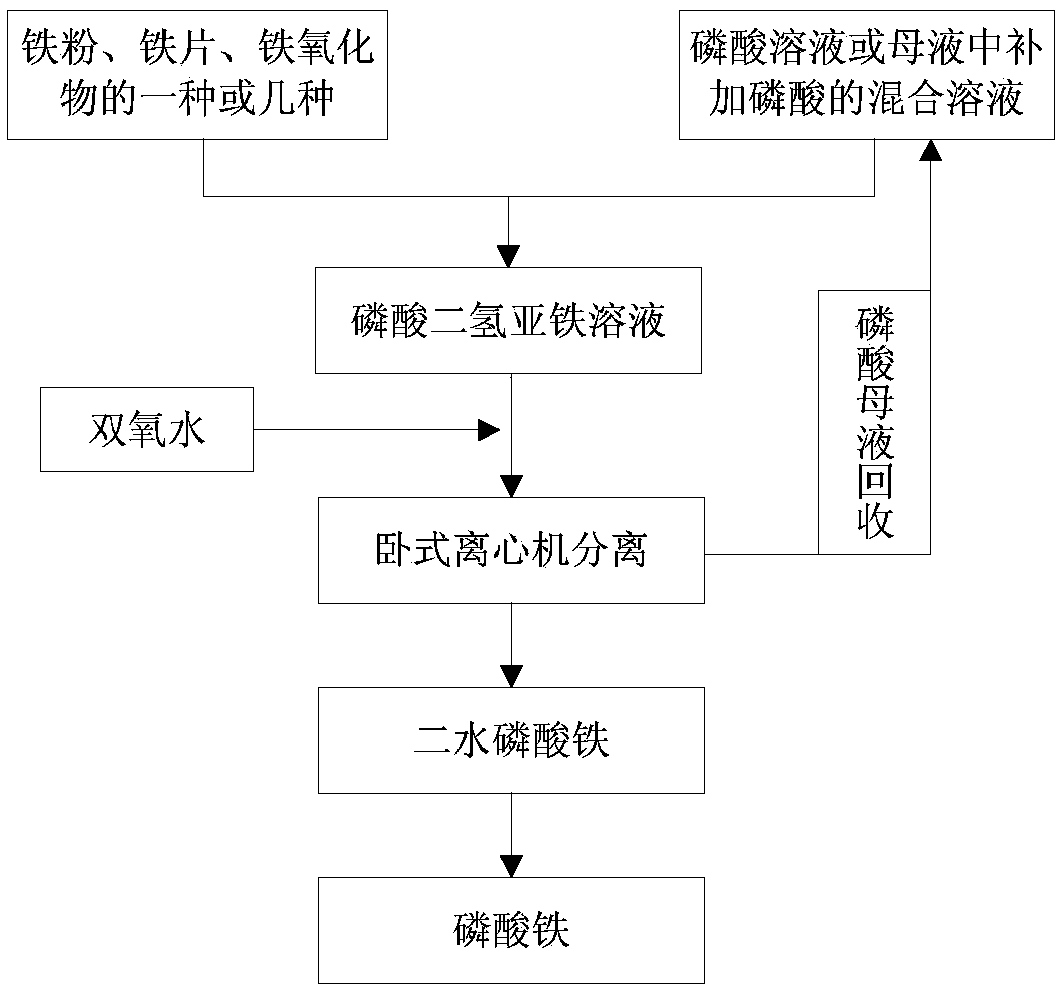
[0030] refer to figure 1 , the preferred embodiment of the present invention provides a kind of preparation method of ferric phosphate, comprises the following steps:
[0031] A preparation method for iron phosphate, comprising the following steps:
[0032] (1) Mix the iron source and the phosphorus source, heat the reaction to obtain a reaction solution, and control the pH value of the reaction end point to 1.8 to 2.5; the iron source is one or more of iron powder, iron flakes, and iron oxides;
[0033] (2) adding hydrogen peroxide to the reaction solution obtained in step (1), after the reaction was completed, the temperature was raised to 85-98° C. to continue the reaction to obtain ferric phosphate dihydrate;
[0034] (3) Calcining the ferric phosphate dihydrate obtained in step (2) to obtain ferric phosphate.
[0035] The above-mentioned preparation method of iron phosphate directly uses one or more of iron powder, iron flakes, and iron oxides as raw materials. Since th...
Embodiment 1
[0054] The preparation method of ferric phosphate in the present embodiment, comprises the following steps:
[0055] (1) Weigh 1613.5g of 85% phosphoric acid solution in the reactor, add water to adjust its concentration to 3.5mol / L, raise the temperature of the solution to 55°C, slowly add 224g of iron powder into the reactor, at this time the iron The molar ratio of source and phosphorus source is 1:3.5, and then add appropriate amount of water to make the mass percentage of iron 5%, and react for 4 hours under constant stirring to obtain a ferrous dihydrogen phosphate solution with a pH value of 1.8, which is filtered.
[0056] (2) Slowly add 755.5g of hydrogen peroxide with a mass fraction of 27% in the above-mentioned ferrous dihydrogen phosphate solution, under constant stirring, the reaction temperature is controlled at 40°C, and the time for adding the hydrogen peroxide is controlled to be 4h; The ions are slowly oxidized, the number of crystal nuclei is controlled, th...
Embodiment 2
[0060] The preparation method of ferric phosphate in the present embodiment, comprises the following steps:
[0061] (1) In the phosphoric acid mother liquor that obtains in embodiment 1, add the phosphoric acid solution of 230.6g 85% again, solution temperature is raised to 55 ℃, 224g iron powder is slowly added in the reactor, now iron source and phosphorus source The mol ratio is 1: 3, then add appropriate amount of water, make the mass percentage content of iron be 5%, react 4h under constant stirring, add the hydrogen peroxide that the mass percentage of 50g is 27%, hydrogen peroxide oxidizes part of ferrous iron to trivalent iron Valence iron, trivalent iron and a small amount of insoluble iron powder undergo oxidation-reduction reactions, thereby promoting the complete reaction of iron powder and converting it into ferrous dihydrogen phosphate to obtain a ferrous dihydrogen phosphate solution with a pH value of 2.2, which is then filtered.
[0062] (2) Slowly add 755.5 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tap density | aaaaa | aaaaa |
| Compaction density | aaaaa | aaaaa |
| Compaction density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com