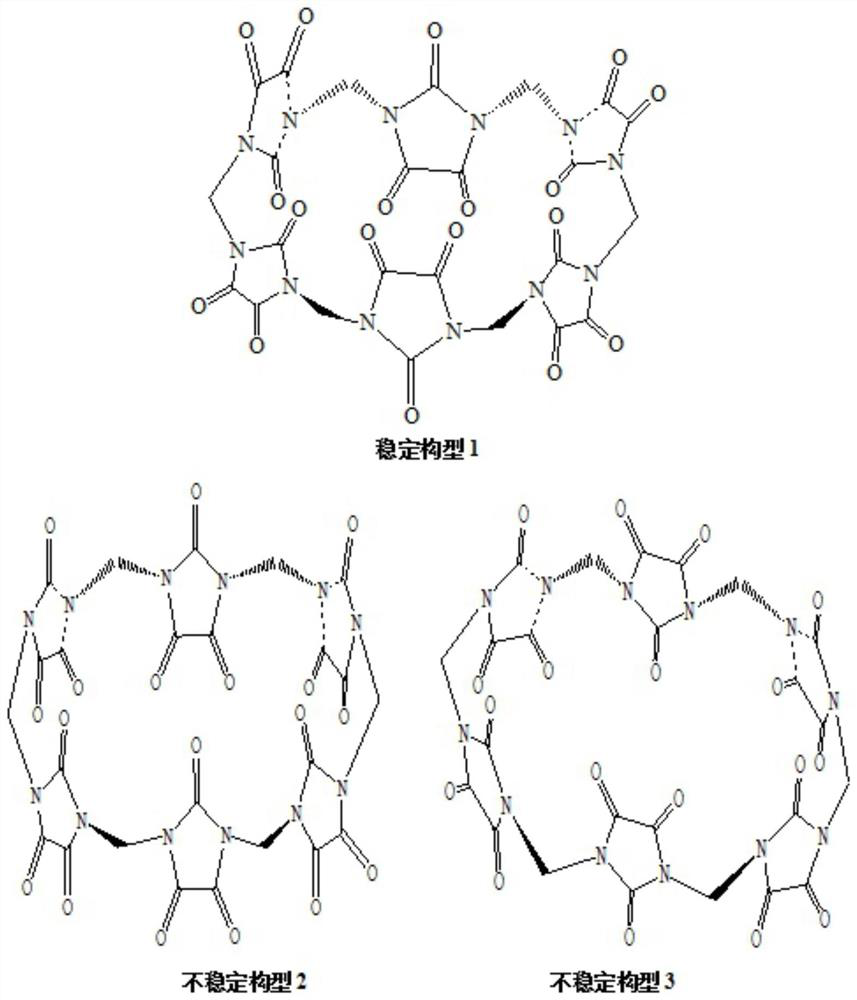
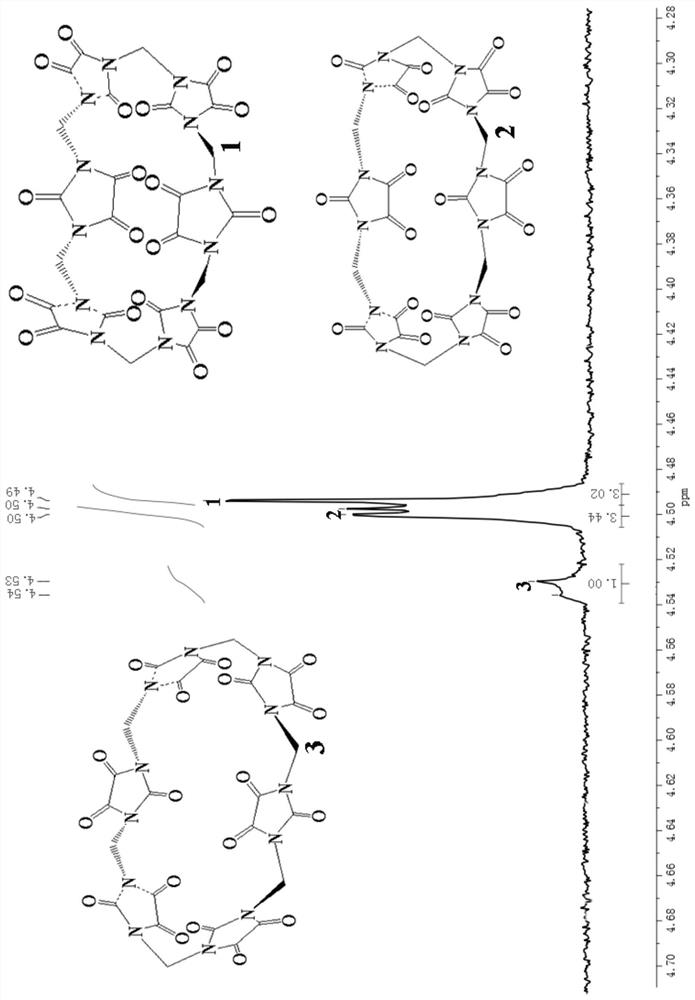
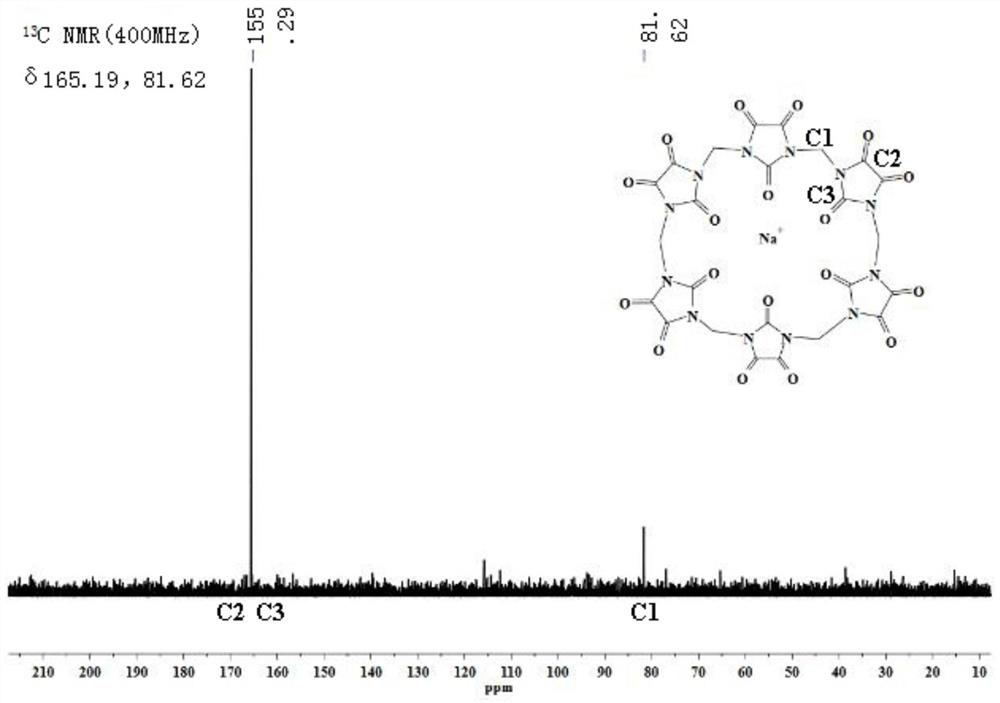
A compound with polycarbonyl substituted six-membered semicucurbitan ring and its preparation method
A polycarbonyl semi-compound technology, applied in the field of cyclic compounds and their synthesis, can solve problems such as the inability to coordinate metal ions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1: Dissolve 1.14g (10mmol) of imidazolidinone in 50ml of water. At this time, the solution was acidic (pH≈2), and anhydrous sodium carbonate solid (0.7g) was added to adjust the pH of the solution to neutral. Then add 0.60g (20mmol) of paraformaldehyde, and the reaction temperature is 55°C. When the paraformaldehyde sinking at the bottom of the flask is observed to disappear, 1.14g (10mmol) of imidazolidinone is added thereto and reacted for 48h. Suction filtration, the filter cake was washed with water until the pH value of the filtrate was neutral, and a solid product was obtained. It was dried and weighed, the product quality was 0.64g, and the yield was 25.40%.
Embodiment 2
[0016] Example 2: 2.28 g (20 mmol) of the prepared imidazolidinone was dissolved in an appropriate amount of water, and 0.60 g (20 mmol) of paraformaldehyde in an equimolar amount was added to react at 55° C. After one day of reaction, the solution in the flask gradually turned into a suspension, and a small amount of concentrated hydrochloric acid was added to adjust its pH to 1, and the reaction was continued for 12 hours. After filtration, the filtrate was spin-dried on a rotary evaporator to obtain a viscous substance, which was added with absolute ethanol to make it no longer viscous to obtain the product, which was dried and weighed to obtain 0.30 g with a yield of 11.88%.
Embodiment 3
[0017] Example 3: Get 2.28g (20mmol) of the prepared imidazolintriketone and dissolve it in an appropriate amount of water, add anhydrous sodium carbonate solid (about 1.4g) to adjust the pH value of the solution to neutrality, add 0.60g (20mmol) equimolar amount of paraformaldehyde, react at 55°C. Reaction 48h. Suction filtration, the filter cake was washed with water until the pH value of the filtrate was about neutral, and a solid product was obtained. It was dried and weighed, the product quality was 0.54g, and the yield was 21.43%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com