Tuberculosis vaccine and preparation technology thereof
A preparation process, a technology for tuberculosis, applied in genetic engineering, botanical equipment and methods, biochemical equipment and methods, etc., can solve the problem that it is difficult to obtain the effect of stimulating the activation of specific lymphocytes, cannot kill and clear Mycobacterium tuberculosis, The problem of poor killing effect of latent infectious bacteria can achieve the effect of enhancing killing and clearing of tuberculosis bacteria, enhancing antigen-specific immune response, and improving antigen presentation ability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] A tuberculosis vaccine designed on the basis of Figure 17 As shown, it includes a recombinant lentiviral expression plasmid pLenti-spHL47 / pLenti-spHI1 and lentiviral particles packaged with the recombinant lentiviral expression plasmid. The lentiviral particles are used to carry the above-mentioned lentiviral expression vectors, so that they can efficiently enter cells and express the above-mentioned recombinant target molecules for a long time.
[0056] Among them, pLenti-spHL47 / pLenti-spHI1 includes the following main components:
[0057] (1) Lentiviral framework plasmid: CS-CDF-CG-PRE. Function: used to provide frame information of lentiviral expression vector.
[0058] (2) Macrophage specific promoter: SP146. Function: This is a macrophage-specific promoter, which is used to control the expression of hspX gene and lrg47 / irgm1 gene, so that they can only be expressed in macrophages, but not in other cells.
[0059] (3) hspX gene: an expression gene that specific...
Embodiment 2
[0063] The preparation technology of tuberculosis vaccine of the present invention comprises the following steps:
[0064] 1. Using the plasmid pSP146-GFP as a template, the macrophage-specific promoter SP146 fragment was amplified by PCR technology (primers used: S1 / S2, the sequence is shown in Table 1), and then molecular cloning technology (refer to Sambrook.J editor-in-chief The third edition of "Molecular Cloning Experiment Guide"), the SP146 promoter was cloned into the lentiviral framework plasmid CS-CDF-CG-PRE using restriction enzymes EcoRI and AgeI to replace the CMV promoter in the original plasmid, Obtain macrophage-specific expression lentiviral expression vector pLenti-SP146.
[0065] 2. Use the PCR method (refer to the third edition of "Molecular Cloning Experiment Guide" edited by Sambrook.J) to amplify and obtain the hspX gene specifically expressed during the latent infection period of Mycobacterium tuberculosis (primers used: H1 / H2, the sequence is shown in ...
Embodiment 3
[0073] The identification of the tuberculosis vaccine of the present invention specifically includes the following aspects:
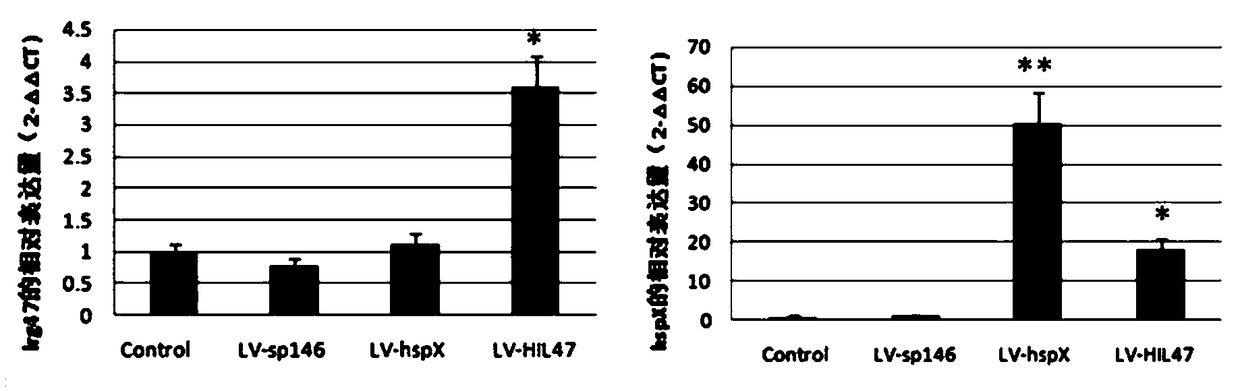
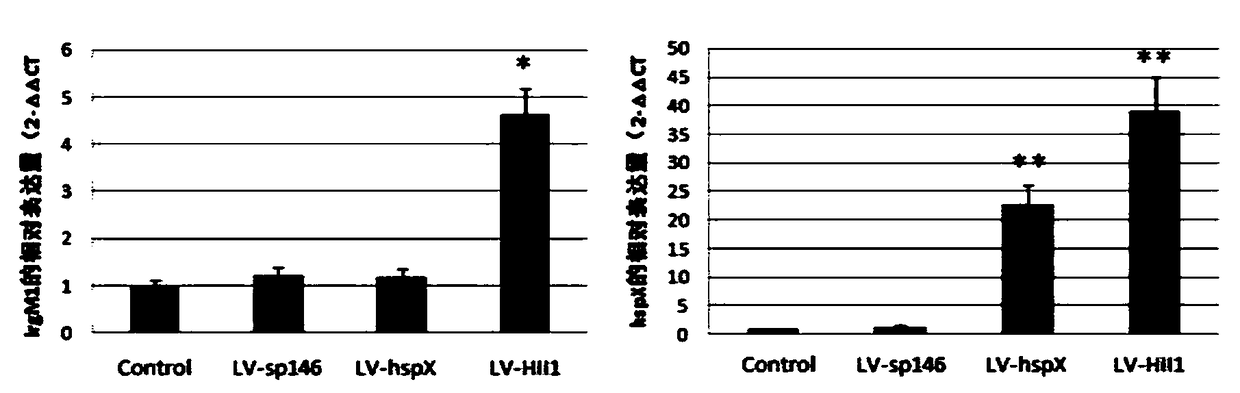
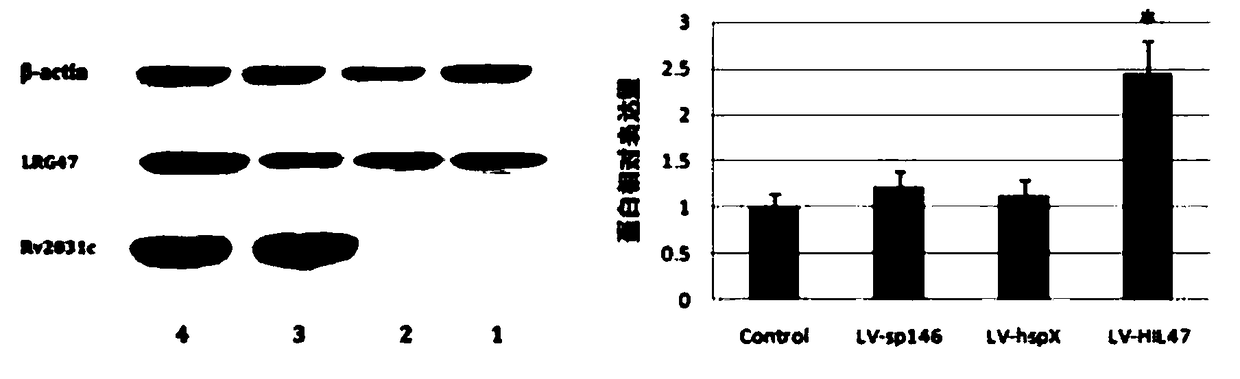
[0074] 1. Expression identification: LV-sp146, LV-hspX, LV-HIL47, and LV-HII1 were used to infect macrophage RAW264.7 cells, and cells not infected with lentivirus were set as controls. The cells in each group were collected 72 hours after infection, and the total RNA and protein of the cells were extracted. The expression levels of hspX and lrg47 / irgm1 were detected by RT-PCR technology, and the expression levels of HSP X and LRG47 / IRGM1 proteins were detected by Western blotting. The results showed that , after LV-hspX, LV-HIL47 and LV-HII1 infected RAW264.7 cells, they can all express the corresponding recombinant proteins effectively in the cells, such as Figure 1-Figure 4 shown. figure 1 RT-PCR was used to detect the mRNA transcription level of the recombinant gene in each lentivirus infection group and control group RAW264.7 cells; figure 2 RT...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com