Preparation and screening of monoclonal antibody against hepatitis B surface antigen
A hepatitis B surface antigen, monoclonal antibody technology, applied in biochemical equipment and methods, chemical instruments and methods, antiviral immunoglobulin, etc., to achieve the effect of improving accuracy, reducing false negative test results, and broad binding ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
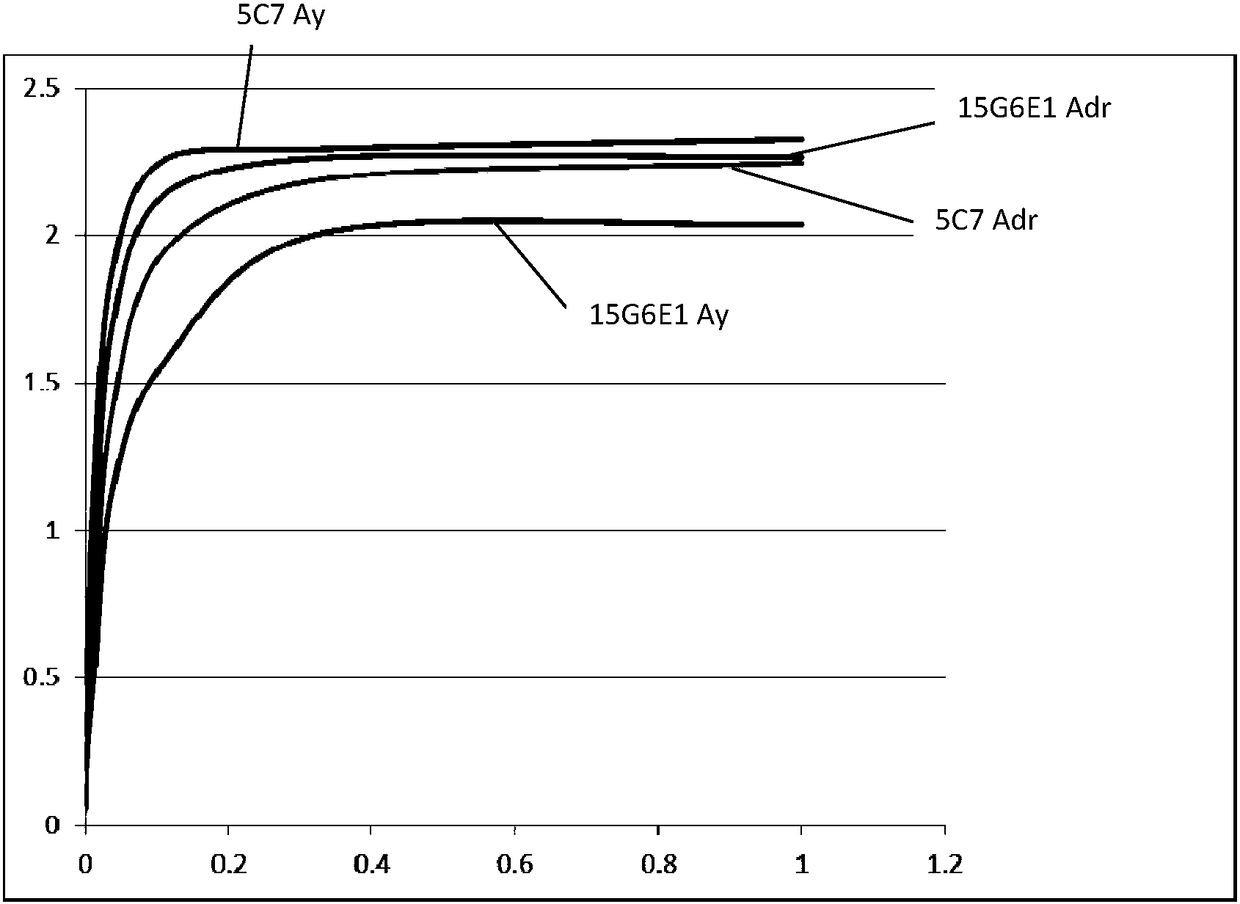
Image
Examples
Embodiment 1
[0048] Example 1: Preparation of hybridoma cell lines (preservation numbers are CCTCC No: C2016198 and CCTCC No: C2016210)
[0049] 1.1 Immunization of animals
[0050] Animal preparation: The immunized animals used to prepare hybridoma cells are not particularly limited, and mice, rats, hamsters, rabbits, and BALB / c mice can be used as immunization objects. In this example, 6-8 week old female BALB / c mice (purchased from Shanghai Animal Center) were selected for immunization according to the pre-established immunization scheme.
[0051] 1.2 Antigen preparation
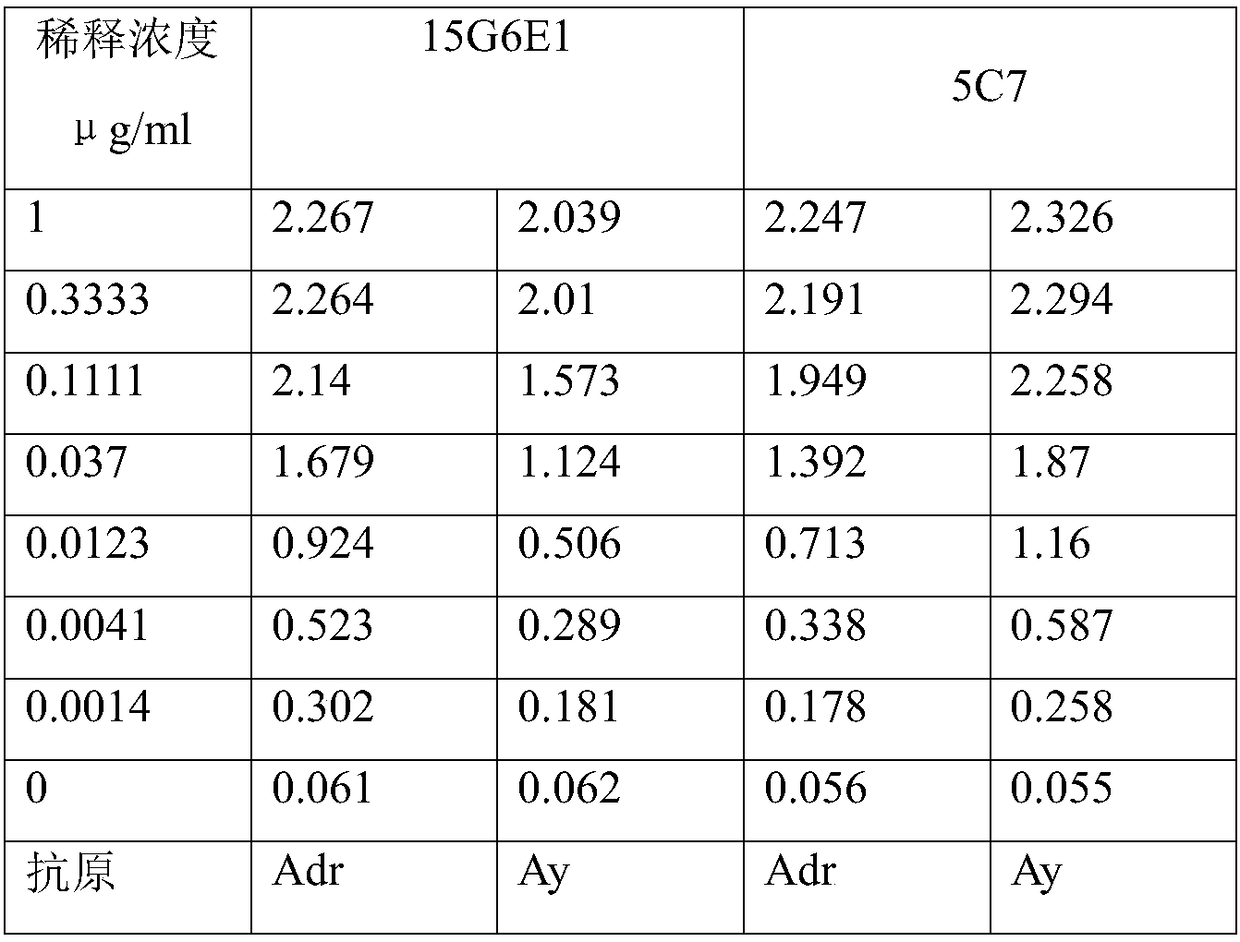
[0052] The two serotypes of HBsAg antigens (purchased from MP Biomedicals Asia Pacific Pte Ltd) are Adr and Ay respectively, Adr is a recombinant protein expressed in Escherichia coli, and Ay is purified from biological samples of HBV-infected patients)
[0053] 1.3 Immunization scheme
[0054] The immunogenicity of a specific antigen is affected by many factors, but the immunogenicity can be enhanced by the use of...
Embodiment 2
[0076] Example 2. Mass production of monoclonal antibodies
[0077] There are mainly two methods for mass production of monoclonal antibodies using hybridoma cells: in vivo and in vitro culture. The cost of culturing hybridoma cells in vitro is relatively high, and experimental animals are not used. Currently, in vivo methods are commonly used to prepare monoclonal antibodies. And the hybridoma cells were injected into the peritoneal cavity of pre-sensitized BALB / c mice.
[0078] The stages of in vivo preparation of monoclonal antibody ascites are as follows:
[0079] (1) Liquid paraffin presensitized mice: the volume of liquid paraffin injected is usually 0.5ml.
[0080] (2) Injection of hybridoma cells: the interval between injection of liquid paraffin and hybridoma cells is optimal at 7-20 days, and the number of injected hybridoma cells is 0.5×10 6 -2×10 6 optimal. Usually, mouse ascites can be formed 7-10 days after injection of hybridoma cells.
[0081] Collection...
Embodiment 3
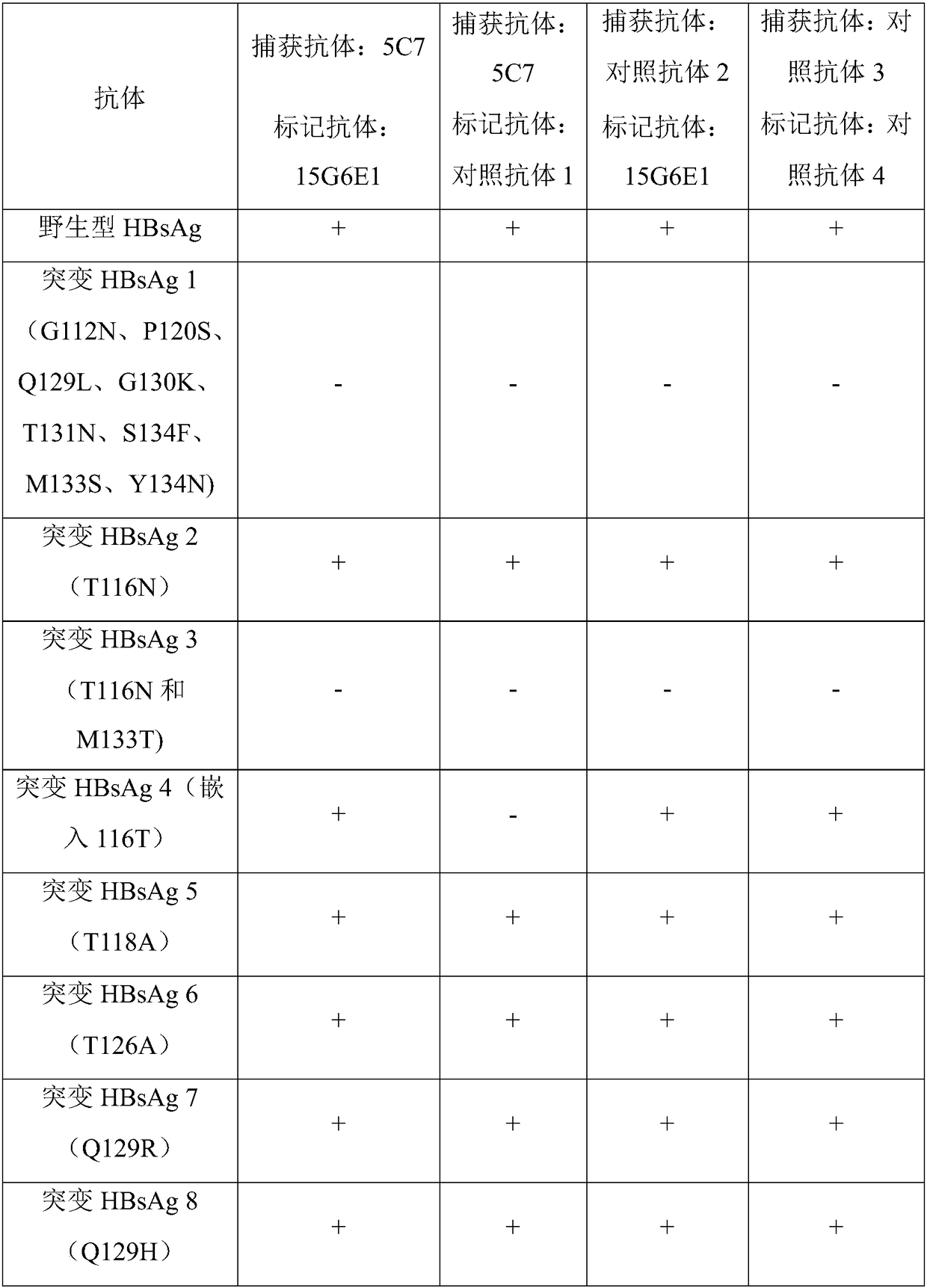
[0082] Example 3. Purification, Identification and Application of Monoclonal Antibodies in Test Kits
[0083] 3.1 Purification of monoclonal antibodies
[0084] The method of purifying monoclonal antibody from ascites is basically the same as the method of purifying immunoglobulin. A salt precipitation method, molecular sieve, etc. may be used. If the antibody is mouse IgG, affinity purification methods such as Protein A and Protein G can also be used.
[0085] Antibodies 15G6E1 and 5C7 in the present invention were purified using Protein A.
[0086] After mixing equal volumes of ascitic fluid and loading buffer, load the sample on the Protein A column that has been equilibrated with loading buffer. After loading the sample, use 0.01M PBS solution to elute the impurity protein, and stop the elution when the OD280 of the effluent is <0.03. Then use the eluent to elute the target antibody, and collect the effluent. When collecting, add hydrochloric acid solution dropwise to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com