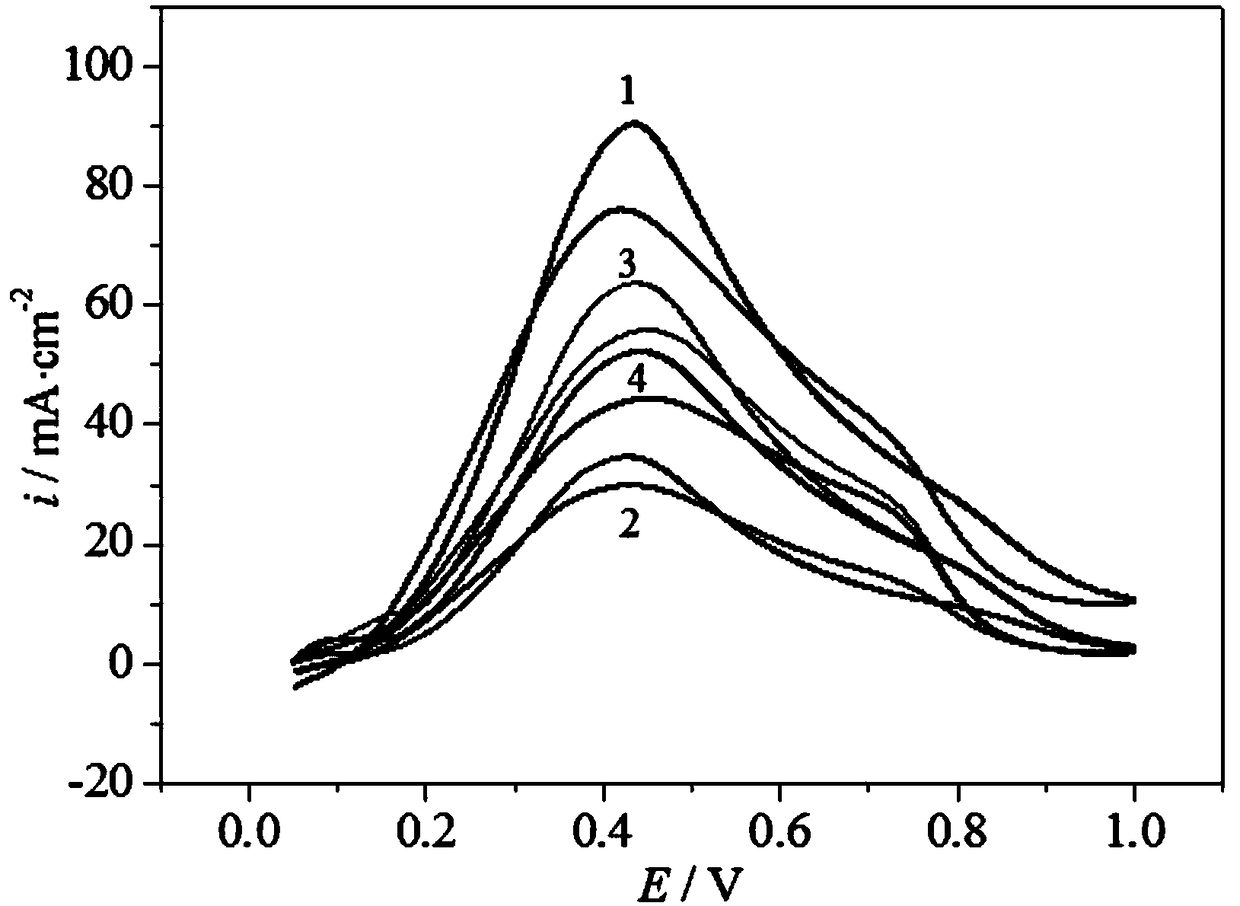
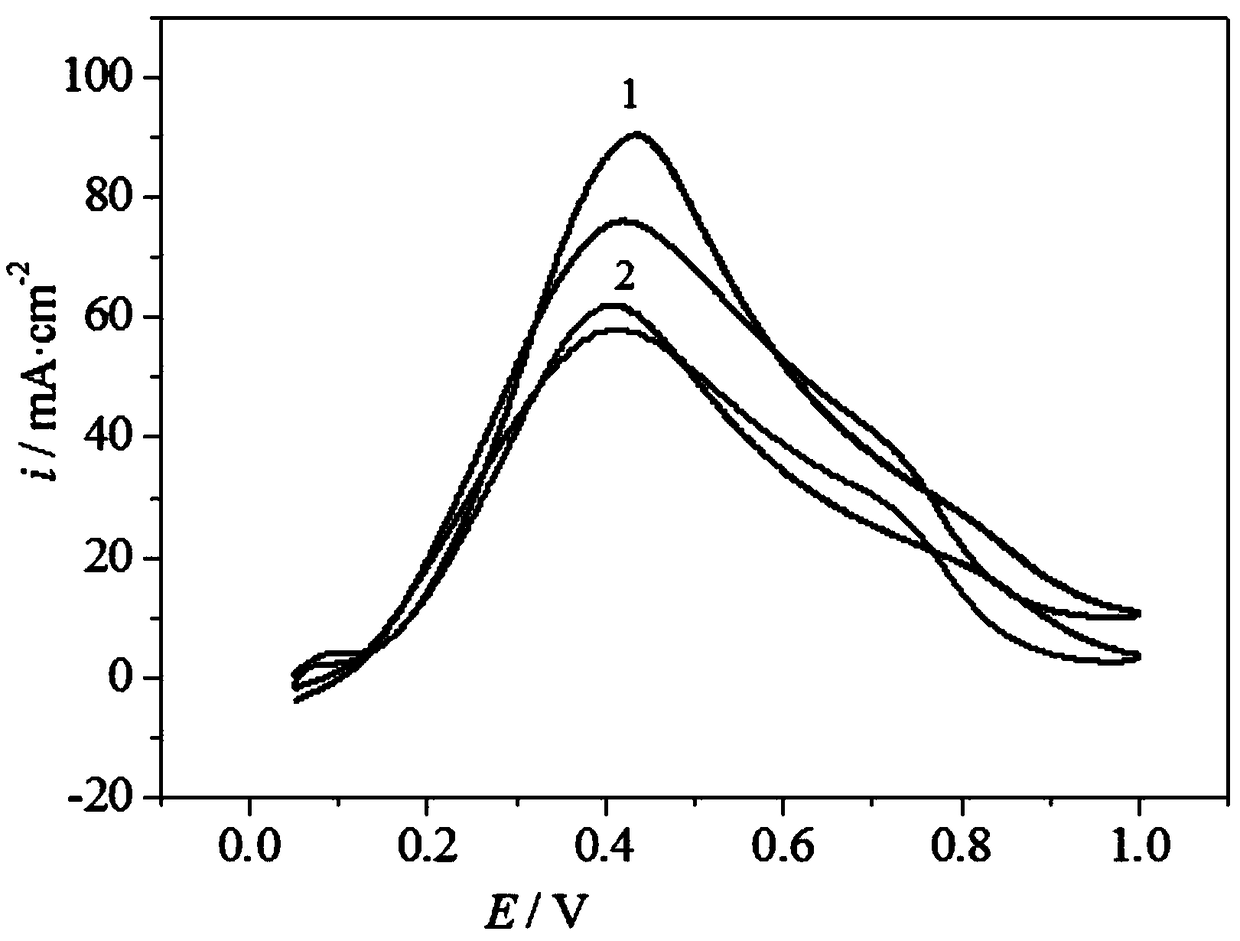
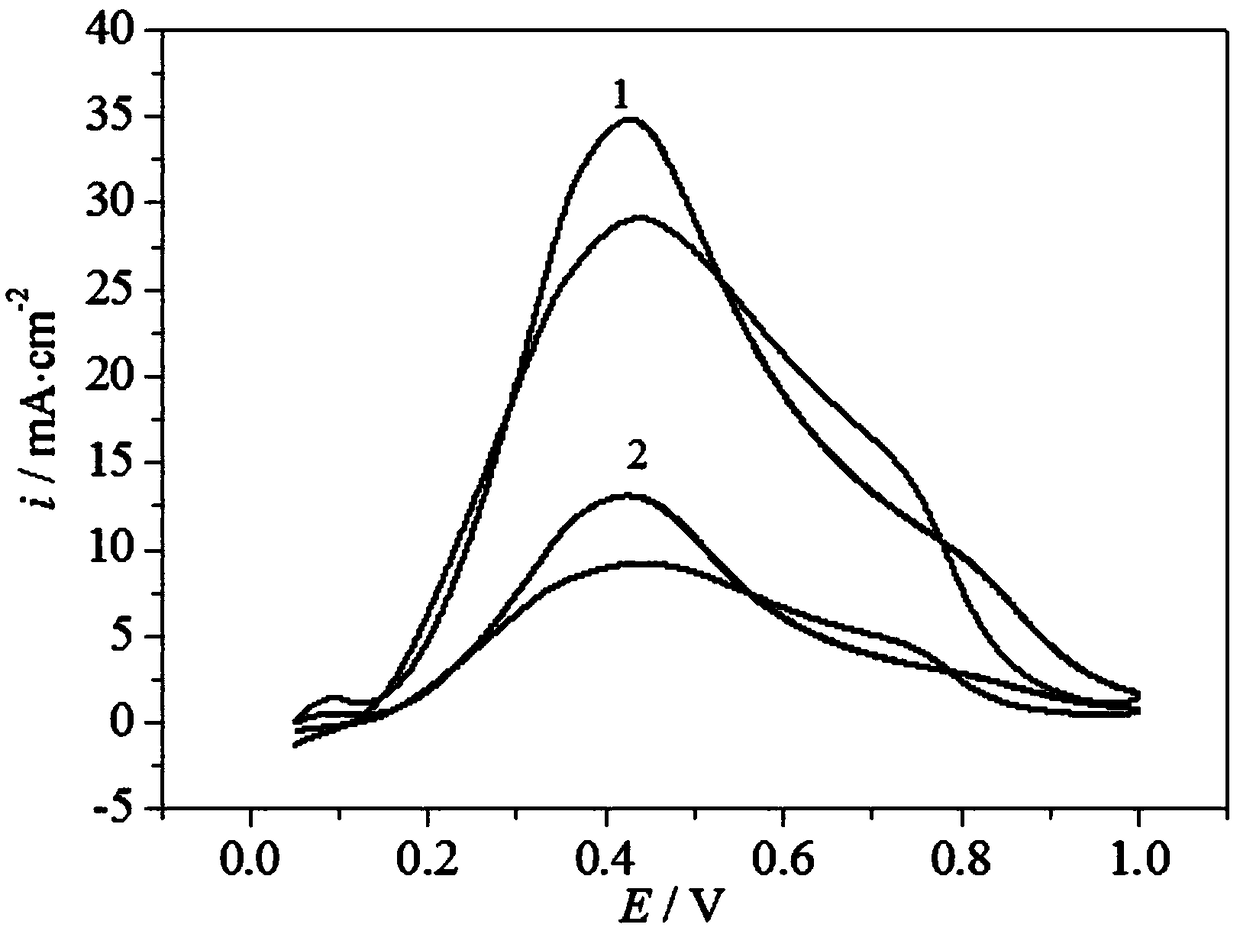
Palladium-based catalyst carrier for direct formic acid fuel cell and preparation method thereof
A formic acid fuel cell and catalyst carrier technology, applied in fuel cells, battery electrodes, electrochemical generators, etc., can solve the problems of poor interaction between carriers and active metal particles, insufficient stability of Pd catalysts, poor stability of carrier materials, etc. , to achieve the effects of reducing the loading of precious metals, making the preparation method simple and feasible, and improving the corrosion resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0023] Specific embodiment 1: A palladium-based catalyst carrier for a direct formic acid fuel cell in this embodiment is to add a precursor of tungsten, an intermediate of cerium and a carbon material into a solvent, and then undergo ultrasonic dispersion, drying, calcination and grinding to obtain Carrier WO 3 -CeO 2 @C; the precursor of tungsten is ammonium tungstate, ammonium paratungstate or ammonium metatungstate;
[0024] The intermediate of cerium is specifically prepared according to the following steps: the precursor of cerium is added to water to obtain a saturated solution of the precursor of cerium, and the saturated solution of the precursor of cerium is adjusted by using ammonia water with a mass percentage of 9% to 11%. The pH value of the cerium is 8-12, and then under stirring conditions, the temperature of the precursor saturated solution of cerium with a pH value of 8-12 is heated to 50°C-90°C, and the reaction is carried out at a temperature of 50°C-90°C ...
specific Embodiment approach 2
[0029] Embodiment 2: The difference between this embodiment and Embodiment 1 is that the cerium salt is cerium nitrate or cerium sulfate. Others are the same as in the first embodiment.
specific Embodiment approach 3
[0030] Specific embodiment three: the difference between this embodiment and specific embodiment one or two is that the precursor of cerium is added to water to obtain a saturated solution of the precursor of cerium, and the precursor of cerium is adjusted by using ammonia water with a mass percentage of 10% The pH value of the precursor saturated solution is 10, and then under stirring conditions, the temperature of the precursor saturated solution of cerium with a pH value of 10 is heated to 80 ° C, and the temperature is 80 ° C under the condition of reaction for 3 hours, after aging, filtering, Washing and drying give intermediates of cerium. Others are the same as in the first or second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com