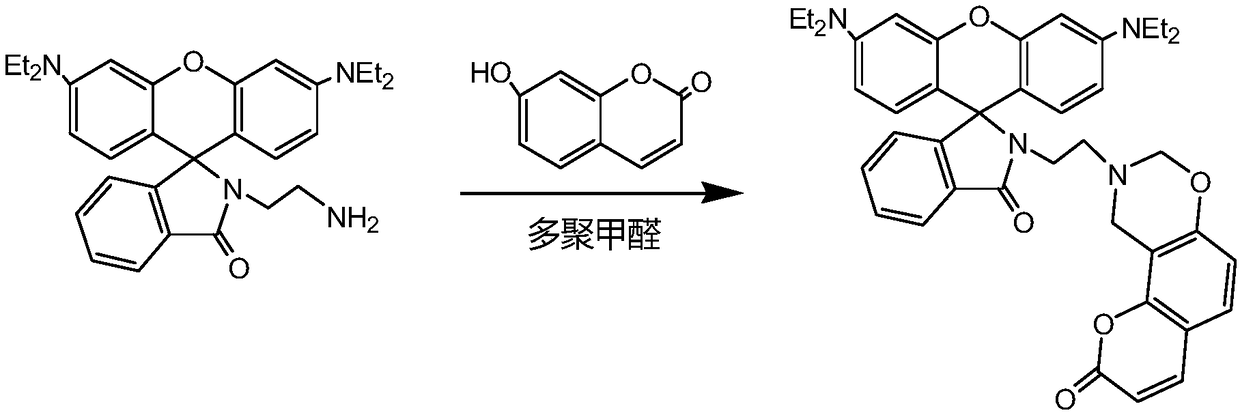
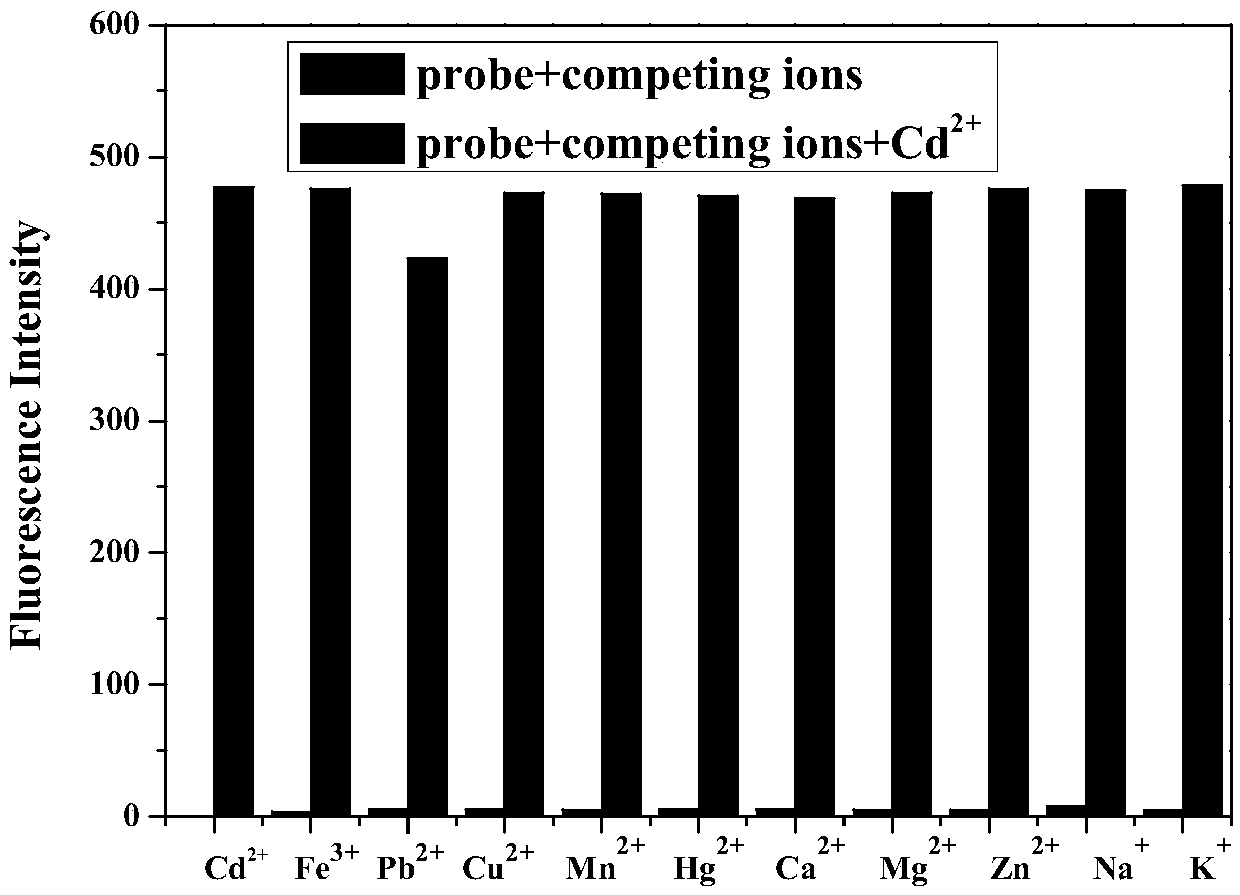Rhodamine-based Cd<2+> fluorescent probe prepared by using Mannich reaction, and synthesis method of rhodamine-based Cd<2+> fluorescent probe
A fluorescent probe and rhodamine-based technology, applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of cumbersome synthesis steps of fluorescent probes, short excitation wavelength, low sensitivity, etc., and achieve mild reaction conditions, Simple operation and good anti-interference ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Step 1: Synthesize rhodamine lactam intermediate, weigh 2g of rhodamine B dye, dissolve it in ethanol solvent, then add ethylenediamine solution dropwise into rhodamine B solution, heat to 50°C, and reflux for 6h , the solution changes from maroon red to orange yellow during the reaction process, and the rhodamine B lactam intermediate is obtained;
[0029] Step 2: Synthesis of rhodamine base Cd 2+ As a fluorescent probe, rhodamine lactam (1.0mmol), paraformaldehyde (1.2mmol), and 7-hydroxycoumarin (1.5mmol) were dissolved in an organic solvent, heated to 80°C under the protection of an inert gas, and refluxed. TLC monitors the reaction process, and the reaction can be stopped until the raw material point no longer disappears. The reaction solution is cooled to room temperature, the solvent is evaporated under reduced pressure, and CHCl is added. 3 Dissolved to obtain a clear solution, and then 5% Na 2 CO 3 The solution was washed three times with water, dried over a...
Embodiment 2
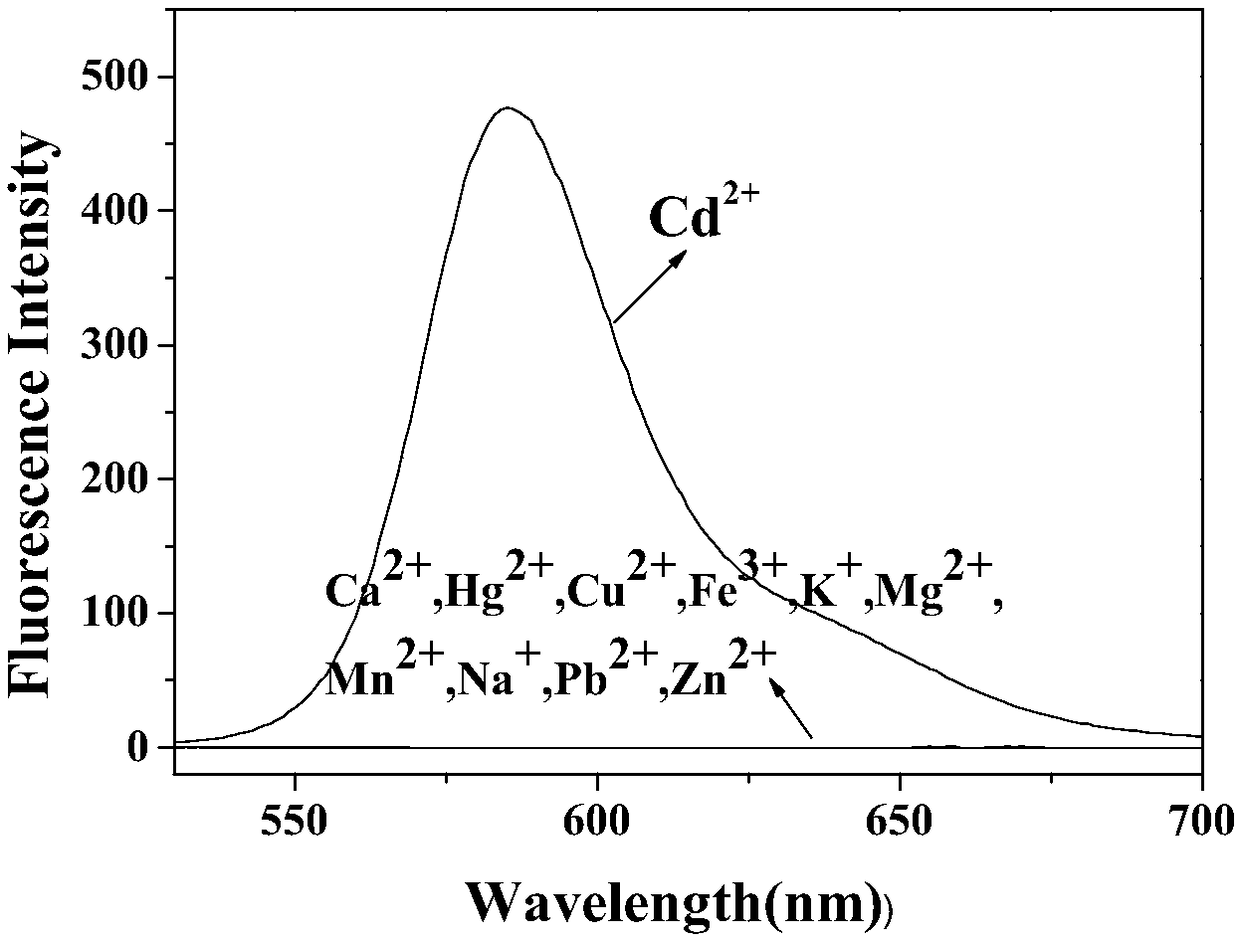
[0031] Such as figure 2 As shown, add 5 times the equivalent of K to the probe solution + 、Na + , Ca2+ , Mg 2+ , Zn 2+ , Hg 2+ , Mn 2+ , Fe 3+ 、Cu 2+ , Pb 2+ When ionized, the probe solution is colorless, and the fluorescence intensity at 580nm does not change. It can be seen that the probe maintains its original structure and can exist stably in these solution systems. When Cd is added to the probe solution 2+ After that, the solution changed from colorless to red, and the fluorescence intensity at 580nm was obviously enhanced, and a strong emission peak appeared. This is due to Cd 2+ Reaction with the probe causes the rhodamine helix to open, resulting in fluorescence emission.
Embodiment 3
[0033] Such as image 3 As shown, taking the fluorescence intensity at the position of the maximum emission peak as the ordinate, and the metal ion as the abscissa, first add 5 times the equivalent of interference ions (K + 、Na + , Ca 2+ , Mg 2+ , Zn 2+ , Hg 2+ , Mn 2+ , Fe 3+ 、Cu 2+ , Pb 2 + ), then add 3 times the equivalent of Cd 2+ , the solution turns red immediately from the colorless state, and its fluorescence intensity is tested. It can be seen from the figure that, in the presence of other interfering ions, the probe is not sensitive to Cd 2+ Still have good recognition ability.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com