Nimesulide preparation and preparation method thereof
A preparation and disintegrant technology, applied in the field of nimesulide preparations and preparations thereof, can solve the problems of reducing the drug efficacy of the preparation, restricting the application, etc., and achieve the effects of high drying efficiency, reducing the quality change of the preparation, and fast dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
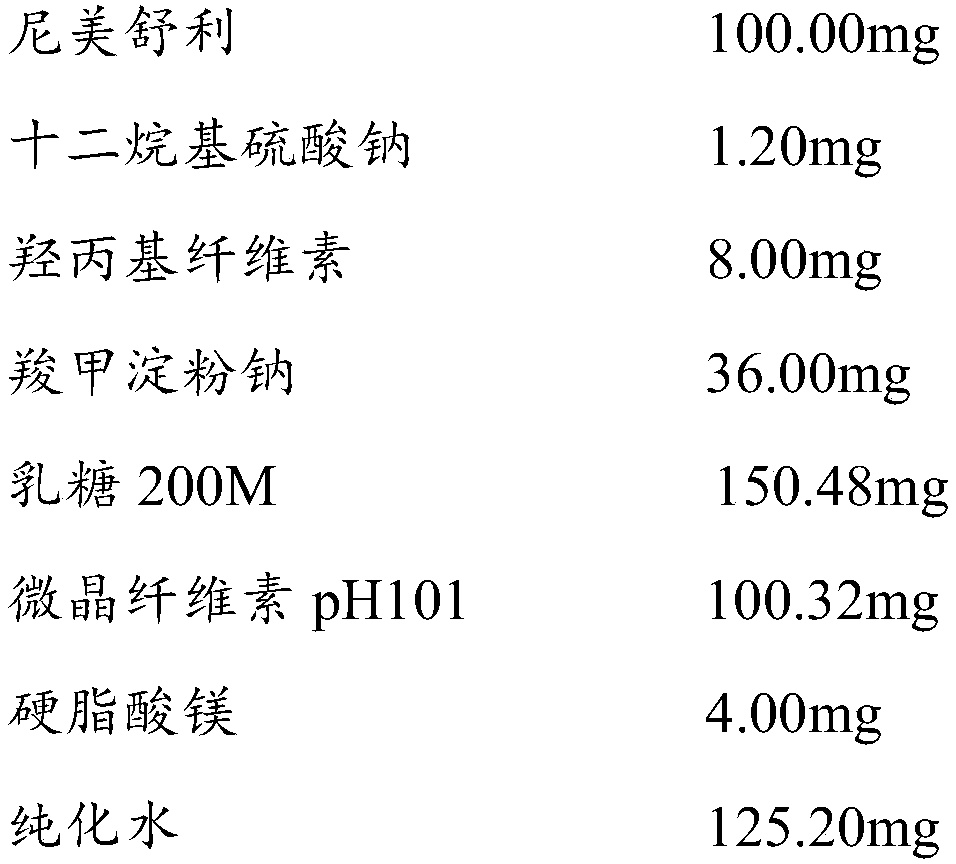
[0065]
[0066] Preparation process: Mix nimesulide raw materials, lactose, microcrystalline cellulose, sodium lauryl sulfate, and sodium carboxymethyl starch internally, add to a wet granulator and stir for 4 minutes to make the mixture even, add hydroxypropyl fiber Stir the plain aqueous solution, 25HZ shear granulation for 4min, wet granules with 24 mesh sieve, granulate with 40 mesh sieve after drying at 60-65℃ until the water content is less than 4.0%, and mix with lubricant and external disintegrating agent, according to the theory Tablet weight for tablet compression. The content uniformity and friability of the plain tablets passed the test.
[0067] The dissolution results of this preparation are shown in Table 1 below.
[0068] Table 1 Example 1 Nimesulide Dispersible Tablets Dissolution Curve Results in Each Medium
[0069] Dissolution medium
Embodiment 2
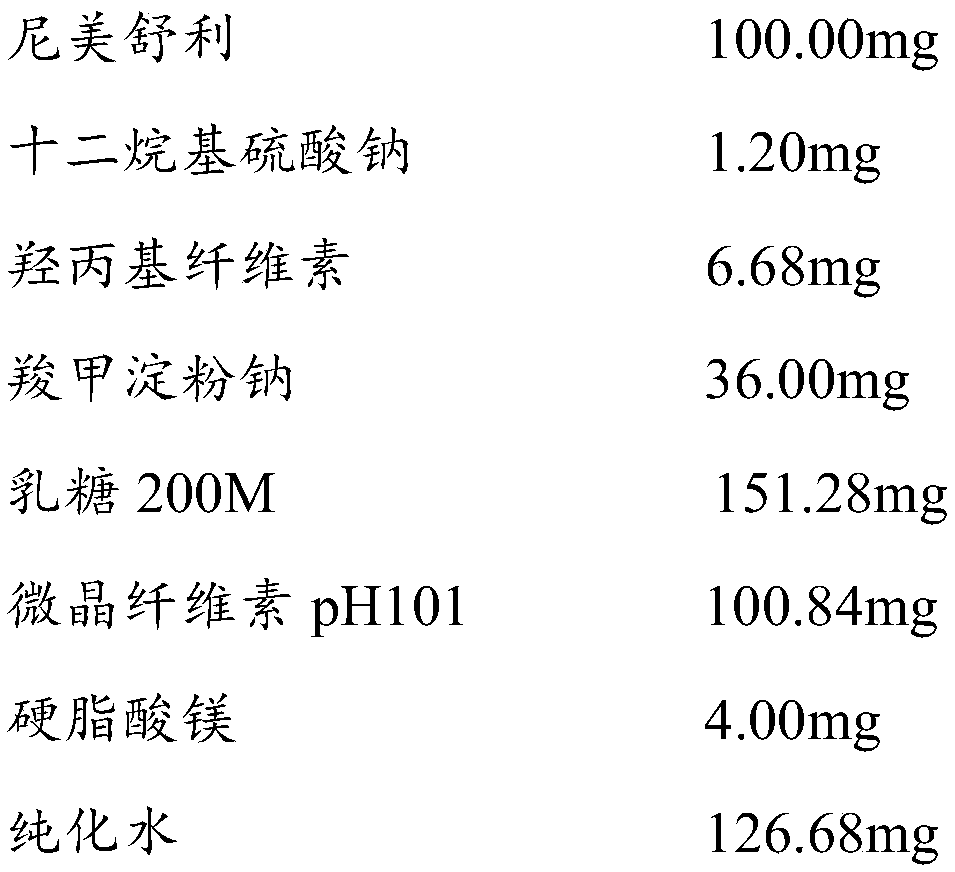
[0071]
[0072] Preparation process: Mix nimesulide raw materials, lactose, microcrystalline cellulose, sodium lauryl sulfate, and sodium carboxymethyl starch internally, add to a wet granulator and stir for 4 minutes to make the mixture even, add hydroxypropyl fiber Stir the plain aqueous solution, 25HZ shear granulation for 4min, wet granules with 24 mesh sieve, granulate with 40 mesh sieve after drying at 60-65℃ until the water content is less than 4.0%, and mix with lubricant and external disintegrating agent, according to the theory Tablet weight for tablet compression. The content uniformity and friability of the plain tablets passed the test.
[0073] The dissolution results of this formulation are shown in Table 2 below.
[0074] Dissolution curve result in each medium of table 2 embodiment 2 nimesulide dispersible tablet
[0075] Dissolution medium
Embodiment 3
[0077]
[0078]
[0079] Preparation process: Mix nimesulide raw materials, lactose, microcrystalline cellulose, sodium lauryl sulfate, and sodium carboxymethyl starch internally, add to a wet granulator and stir for 4 minutes to make the mixture even, add hydroxypropyl fiber Stir the plain aqueous solution, 25HZ shear granulation for 4min, wet granules with 24 mesh sieve, dry at 80-95°C until the water content is less than 4.0%, and then granulate with 40 mesh sieve and mix with lubricant and external disintegrant, according to the theory Tablet weight for tablet compression. The content uniformity and friability of the plain tablets passed the test.
[0080] The dissolution results of this formulation are shown in Table 3 below.
[0081] Dissolution curve result in each medium of table 3 embodiment 3 nimesulide dispersible tablet
[0082] Dissolution medium
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com