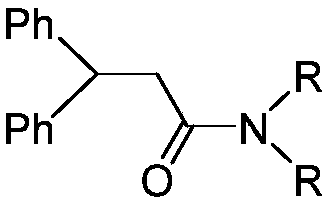
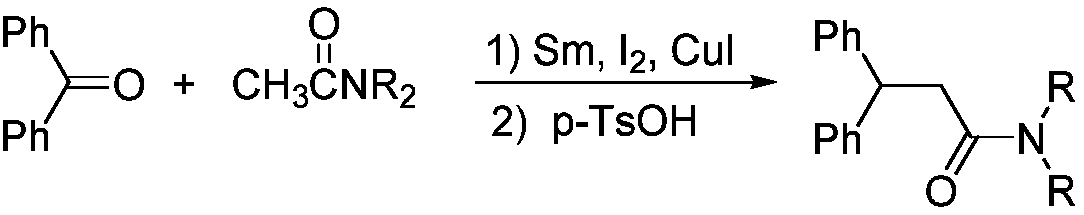Synthesis method of N,N-dialkyl diphenyl propionamide
A technology of diphenylpropanamide and dialkylacetamide, applied in the field of organic chemical synthesis, to achieve the effect of simple and efficient synthetic route, easy separation, and easy reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0019] Add 3.6g of benzophenone and 3.6mL of N,N-dimethylacetamide to the dry reaction vessel in sequence, stir and dissolve, then add 0.9g of newly prepared samarium metal powder, 2.6mg of iodide Cuprous, magnetically stirred for 10min. Add 0.5 mL p-toluenesulfonic acid to the reaction system. The reaction was carried out under slight reflux by heating, and the reaction was 3h. The temperature was lowered, the reaction solution was extracted with ethyl acetate, and the crude product of N,N-dialkyldiphenylpropanamide was obtained after post-processing. Then it was further separated by column chromatography and purified by recrystallization to obtain N,N-dialkyldiphenylpropanamide with a yield of 91%.
[0020] N,N-Dimethyl-3,3-diphenylpropanamide, white solid, melting point 105-106℃. 1 HNMR (500MHz, CDCl 3 )δppm 2.8 3-2.92(s,6H),3.02-3.06(d,2H),4.67-4.73(t,1H),7.16-7.21(m,2H),7.22-7.31(m,8H). 13 C NMR (125MHz, CDCl 3 )δppm 144.3, 128.4, 126.3, 77.3, 77.0, 76.8, 47.0, 39.3...
example 2
[0022] According to the method in Example 1, N,N-diethylacetamide was used instead of N,N-dimethylacetamide, and other conditions remained unchanged, to obtain N,N-diethyldiphenylpropanamide with a yield of 87%.
example 3
[0024] According to the method in Example 1, the reaction temperature is room temperature, the reaction time is 8 h, 3 h, and other conditions remain unchanged, to obtain N,N-dimethyldiphenylpropanamide with a yield of 62%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com