Vildagliptin impurity compound and preparation method thereof, detection method and application
A compound and heterocyclic group technology, applied in the field of vildagliptin impurity compounds and its preparation, can solve problems such as insufficient regulations for highly toxic or genotoxic impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
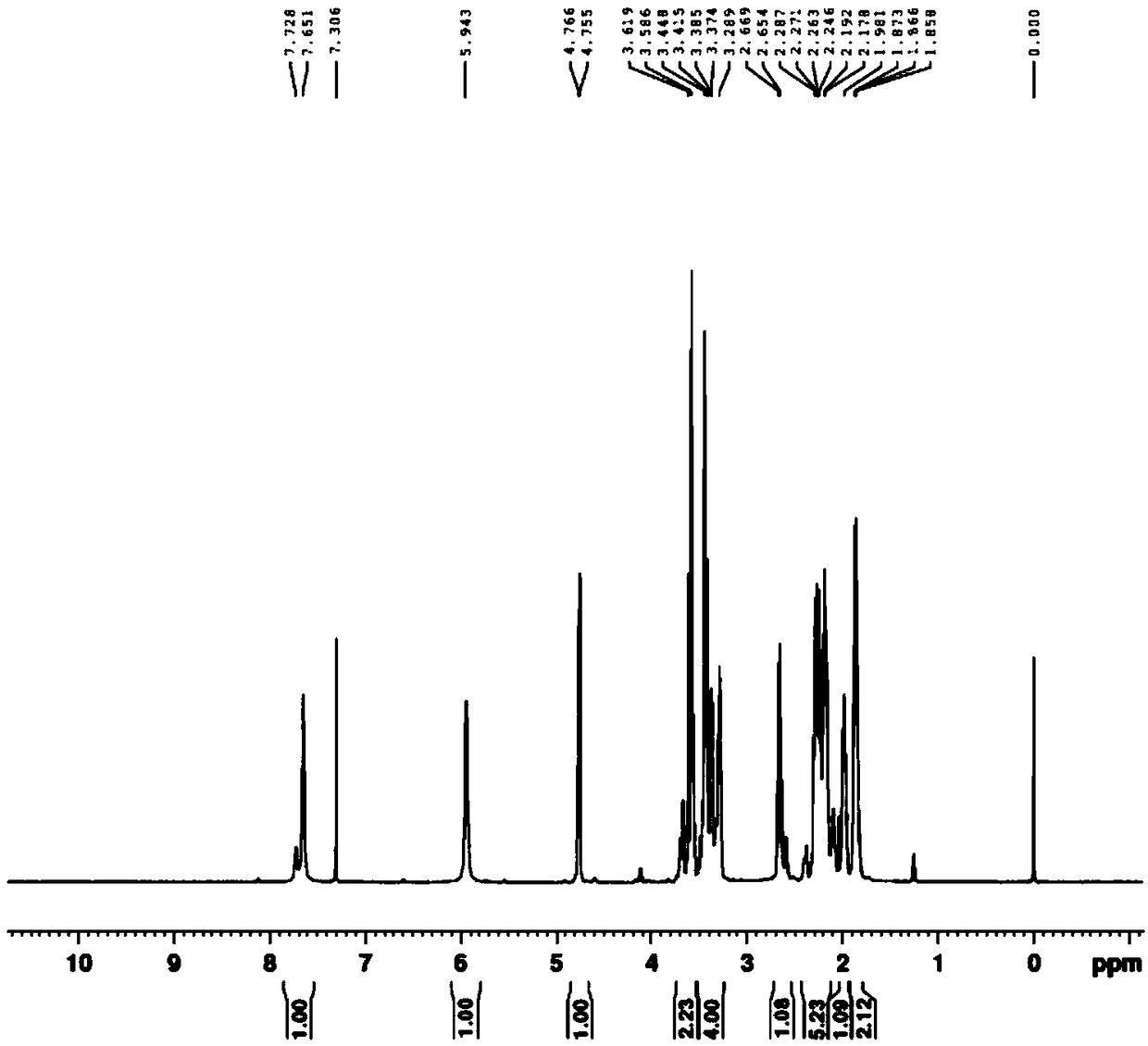
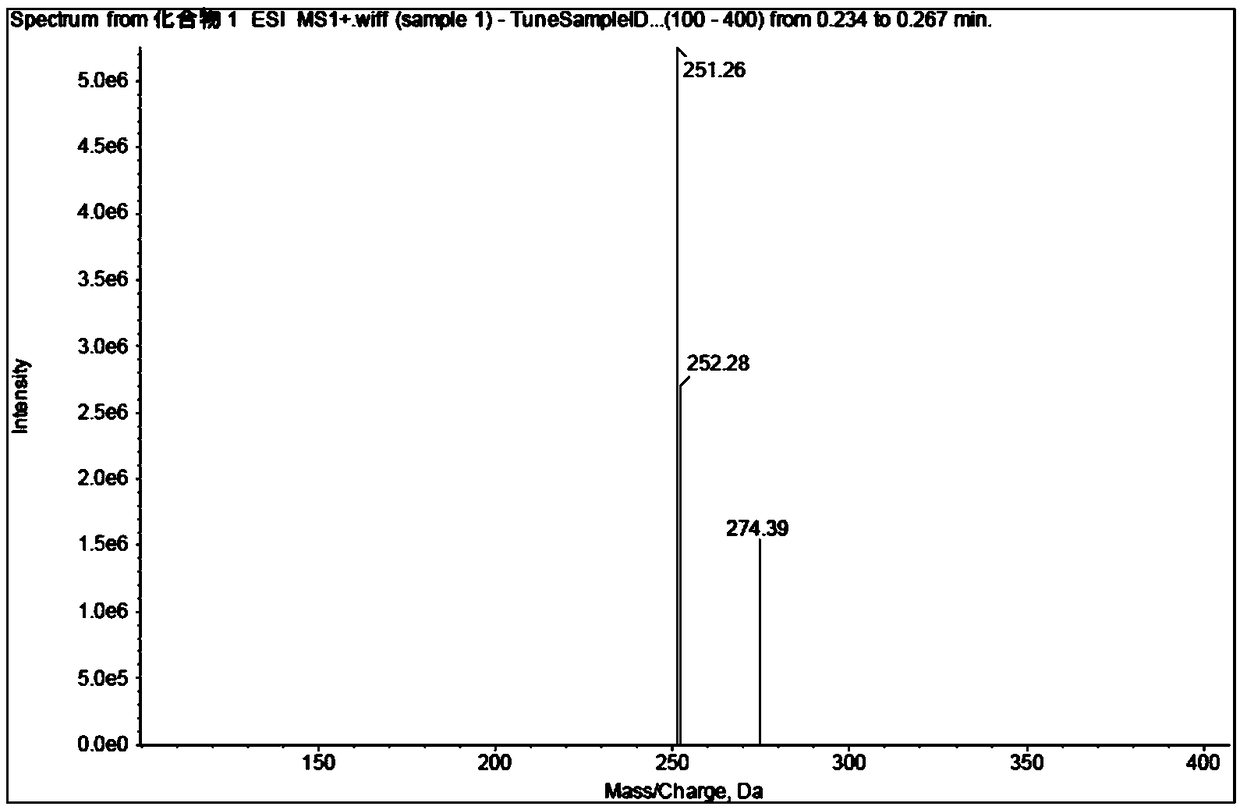
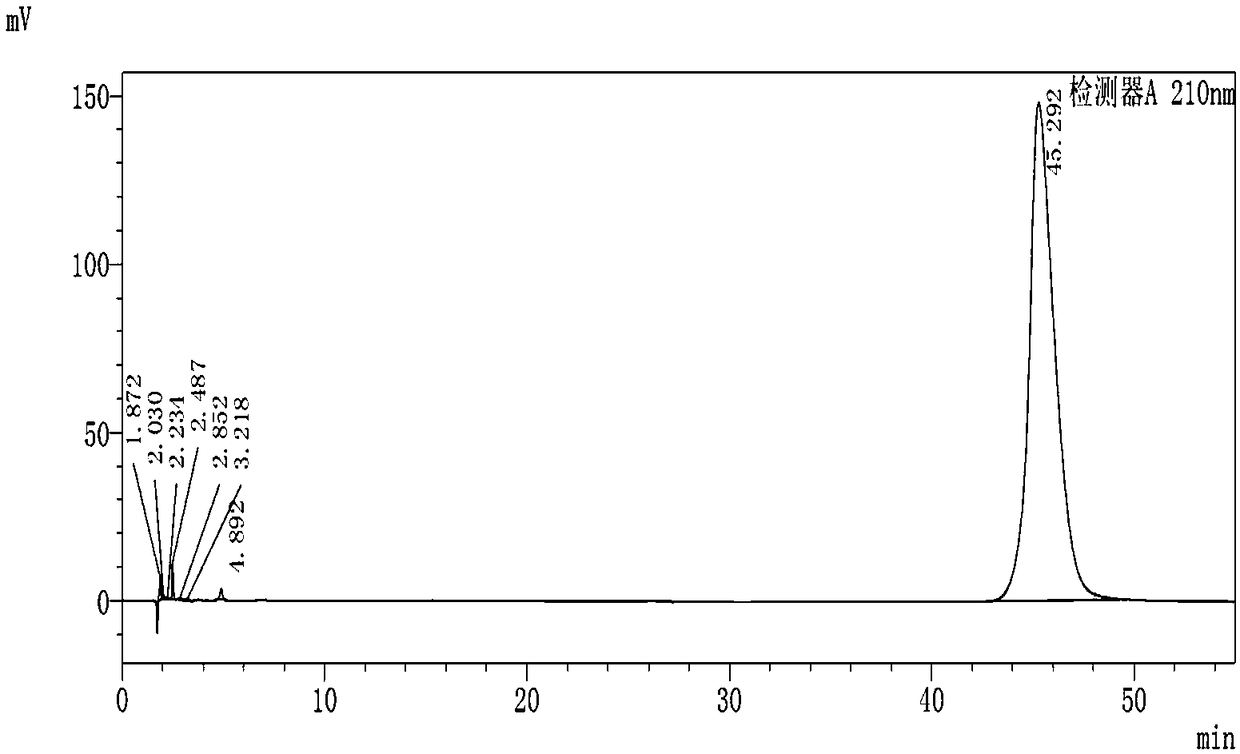
Embodiment 1
[0091] The synthesis of embodiment 1 compound 1
[0092]
[0093] Add 80g of L-prolineamide, 145.3g of potassium carbonate and 1600ml of acetonitrile into a 2000mL reaction flask, stir and lower the temperature to 0-10°C, add dropwise a mixed solution of 102.9g of chloroacetyl chloride and 200ml of acetonitrile within 2 hours, at 0-10°C Insulated and stirred for 2 hours, TLC detected that the reaction was complete, filtered, and washed with 160ml of acetonitrile. The filtrate was concentrated under reduced pressure until the remaining 200-300ml of the reaction solution remained, cooled to -5-10°C, kept stirring for 2 hours, filtered, and washed with 80ml of cold acetonitrile. The filter cake was vacuum-dried at 40-50°C to obtain Intermediate-1107.5g with a yield of 80.5% and a purity of 97.8%.
[0094] Add 100g of Intermediate-1 and 1000ml of tetrahydrofuran into a 2000ml reaction flask, stir and cool down to 0-10°C, add dropwise a mixed solution of 137.7g of trifluoroac...
Embodiment 2
[0101] The synthesis of embodiment 2 compound 2
[0102]
[0103] Add 2.0 g of Compound 1 (prepared from Example 1) and 20 ml of THF into a 250 ml reaction flask, and stir to obtain a light yellow suspension. Control the temperature at 0-10°C, add a solution of 2.1 g of trifluoroacetic anhydride and 20 ml of tetrahydrofuran dropwise, the solid gradually dissolves during the dropwise addition, and a light yellow solution is obtained after the dropwise addition is completed. Insulated and stirred for 1 h, TLC detected that the reaction was complete. The reaction solution was concentrated to near dryness under reduced pressure, and 50ml of ethyl acetate was added to drag it. Add 70ml of ethyl acetate and 500ml of saturated aqueous sodium bicarbonate solution to the residue, stir for 10min, pH 1-2. Adjust the pH value to 7-8 with solid sodium bicarbonate, stir for 0.5 h, retest the pH, and let stand to separate the liquid. The organic phase was retained, and the aqueous ph...
Embodiment 3
[0109] The synthesis of embodiment 3 compound 3
[0110]
[0111] 20g of L-prolineamide and 200ml of THF were added to a 500ml reaction flask, the temperature was controlled to 0-10°C, and a solution of 44.2g of trifluoroacetic anhydride and 50ml of THF was added dropwise. After the addition, keep stirring for 1 h, and TLC detects that the reaction is complete. The reaction solution was concentrated to near dryness under reduced pressure, 300ml of ethyl acetate and 300ml of saturated aqueous sodium bicarbonate were added, stirred for 10min, and the pH was 1-2. Adjust the pH value to 7-8 with solid sodium bicarbonate, stir for 0.5 h, retest the pH, and let stand to separate the liquid. The organic phase was retained, and the aqueous phase was extracted with 300 ml of ethyl acetate. The combined organic phases were added to 300ml saturated aqueous sodium chloride solution, stirred for 1 h, and allowed to stand for phase separation. The organic phase was dried by adding a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com