Pharmaceutical compositions for transmucosal delivery
A composition and drug technology, which can be applied in the directions of drug delivery, active ingredients of hydroxyl compounds, non-active ingredients of polymer compounds, etc., and can solve the problems of not being able to take effect quickly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0074] In another exemplary embodiment, the pharmaceutical composition of the present application is selected from:
[0075] 1) Sumatriptan, Poloxamer 407, NaCMC and mannitol;
[0076] 2) Sumatriptan, Poloxamer 407, NaCMC, Mannitol and KH 2 PO 4 ;
[0077] 3) Sumatriptan, Poloxamer 407, NaCMC, soluble starch and mannitol;
[0078] 4) Sumatriptan, Poloxamer 407, NaCMC, Soluble Starch, Mannitol, Steviolbioside and KH 2 PO 4 ;
[0079] 5) Cannabis resin, poloxamer 407, NaCMC and mannitol;
[0080] 6) Aprepitant, Poloxamer 407, NaCMC and mannitol;
[0081] 7) THC, D-alpha-tocopherol, polyethylene glycol, poloxamer 407, NaCMC, soluble starch, and steviolbioside; and
[0082] 8) Prednisolone, Poloxamer 407, NaCMC, and Mannitol; and
[0083] 9) Insulin, EDTA, Poloxamer 407 and NaCMC.
[0084] In another aspect, the application provides a method of preparing the composition of the embodiment, comprising the steps of:
[0085] 1) dissolving two or more polymers, fast dissolv...
example 1
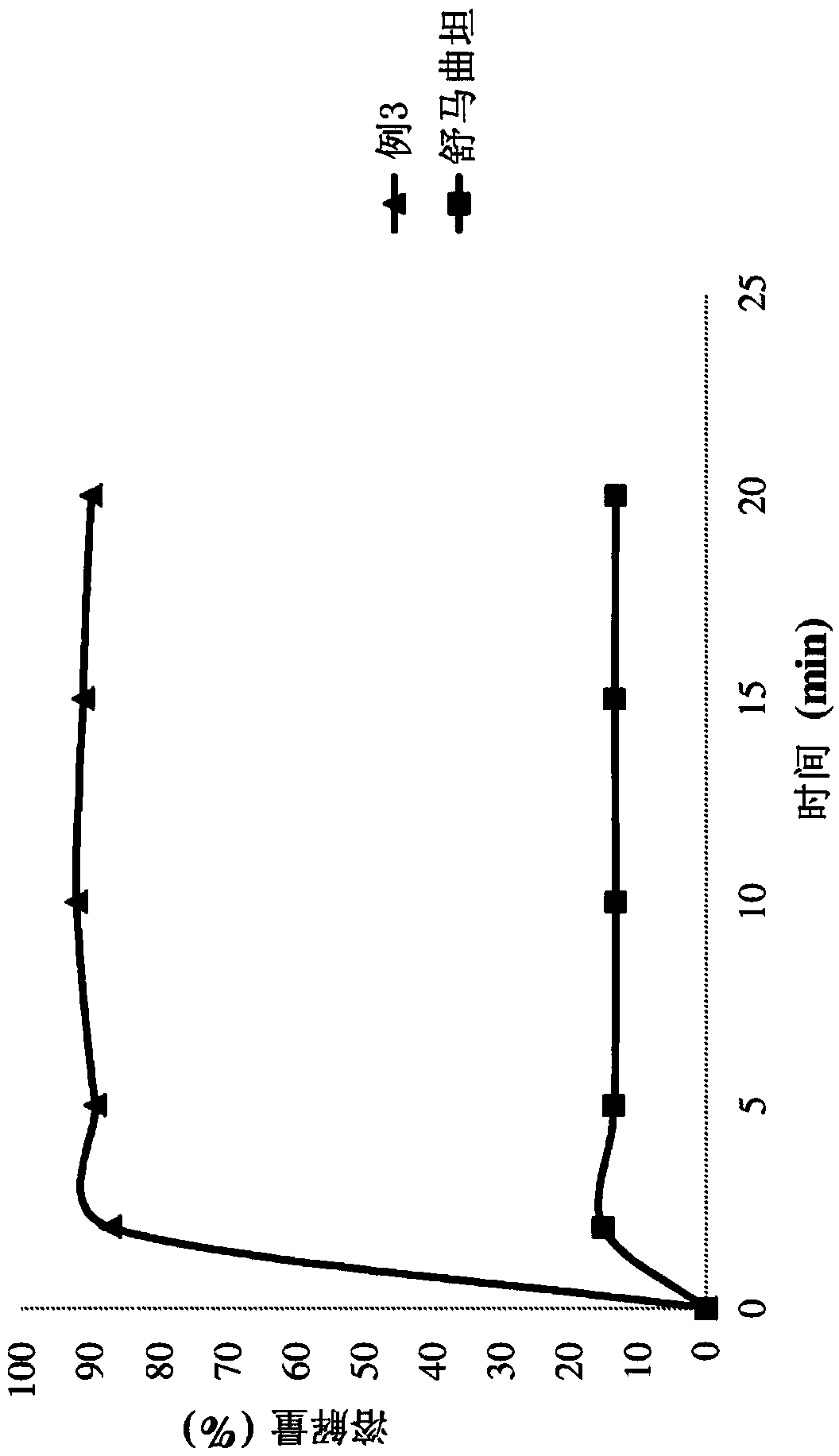
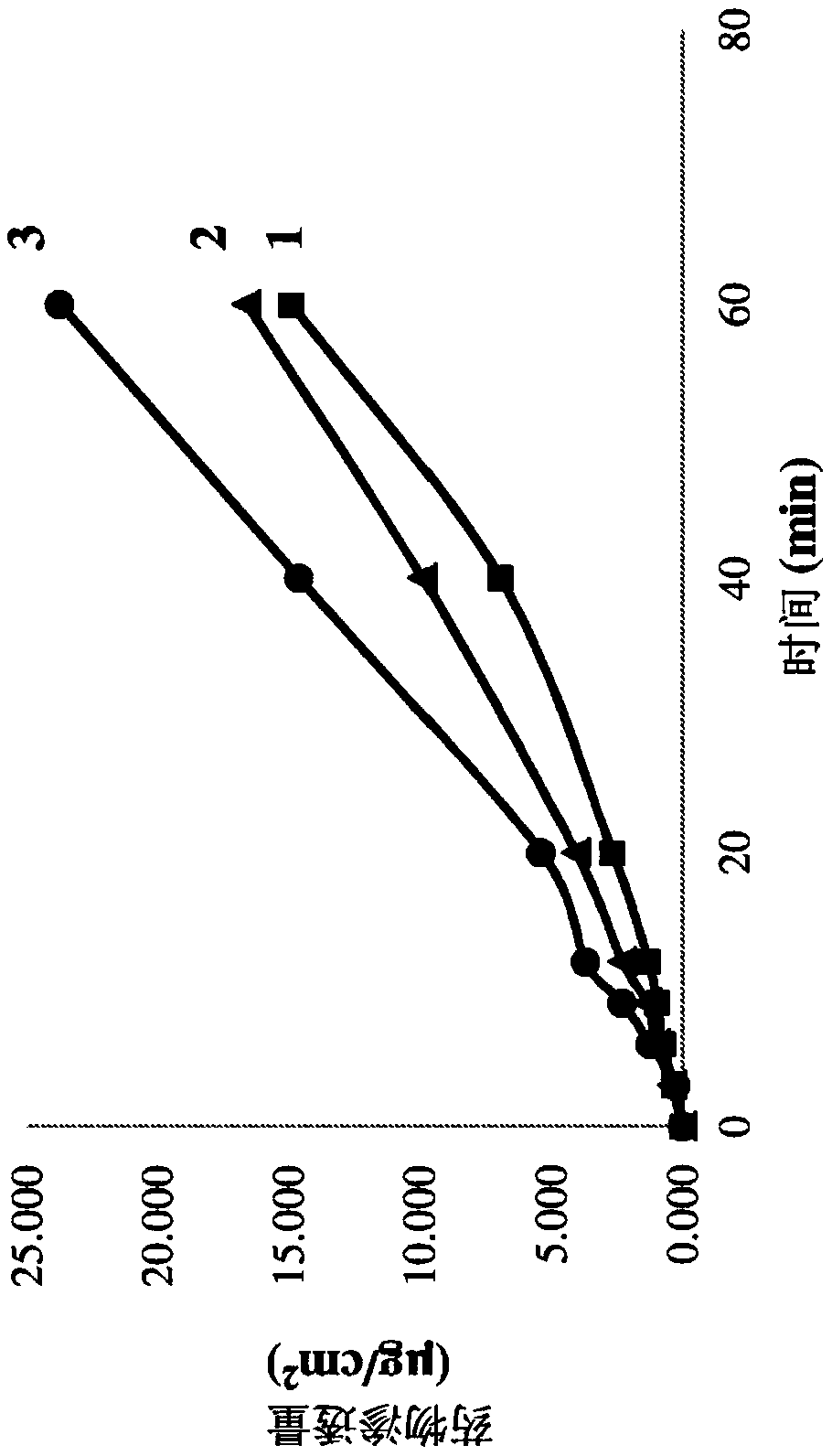
[0160] Example 1: Formulation of sumatriptan with Poloxamer 407 and NaCMC
[0161] Drug solution: sumatriptan (1.0 g) was dissolved in a mixture of 17 g of n-propanol and 8 g of acetone at 25° C. under stirring at 300 rpm.
[0162] Polymer solution: Poloxamer 407 (2.0 g), NaCMC (1.0 g) and mannitol (0.5 g) were dissolved in 50 ml of water at 57°C with stirring at 300 rpm.
[0163] The drug solution was added to the polymer solution at a feed rate of 2 ml / min with stirring at 300 rpm at 55°C. The obtained clear and uniform hot (50-55° C.) solution was spray-dried using a Buchi mini-spray dryer with an inlet air temperature of 105° C. and an outlet air temperature of 62° C. to obtain a powder.
example 2
[0164] Example 2: Formulation of sumatriptan with Poloxamer 407, NaCMC and modified starch
[0165] Drug solution: sumatriptan (1.0 g) was dissolved in a mixture of 17 g of n-propanol and 8 g of acetone at 25° C. under stirring at 300 rpm.
[0166] Polymer solution: Poloxamer 407 (2.0 g), NaCMC (0.5 g), modified starch (0.5 g) and mannitol (0.5 g) were dissolved in 50 ml of water at 57° C. under stirring at 300 rpm.
[0167] The drug solution was added to the polymer solution at a feed rate of 2 ml / min with stirring at 300 rpm at 55°C. The obtained clear and uniform hot (50-55° C.) solution was spray-dried using a Buchi mini-spray dryer with an inlet air temperature of 105° C. and an outlet air temperature of 62° C. to obtain a powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com