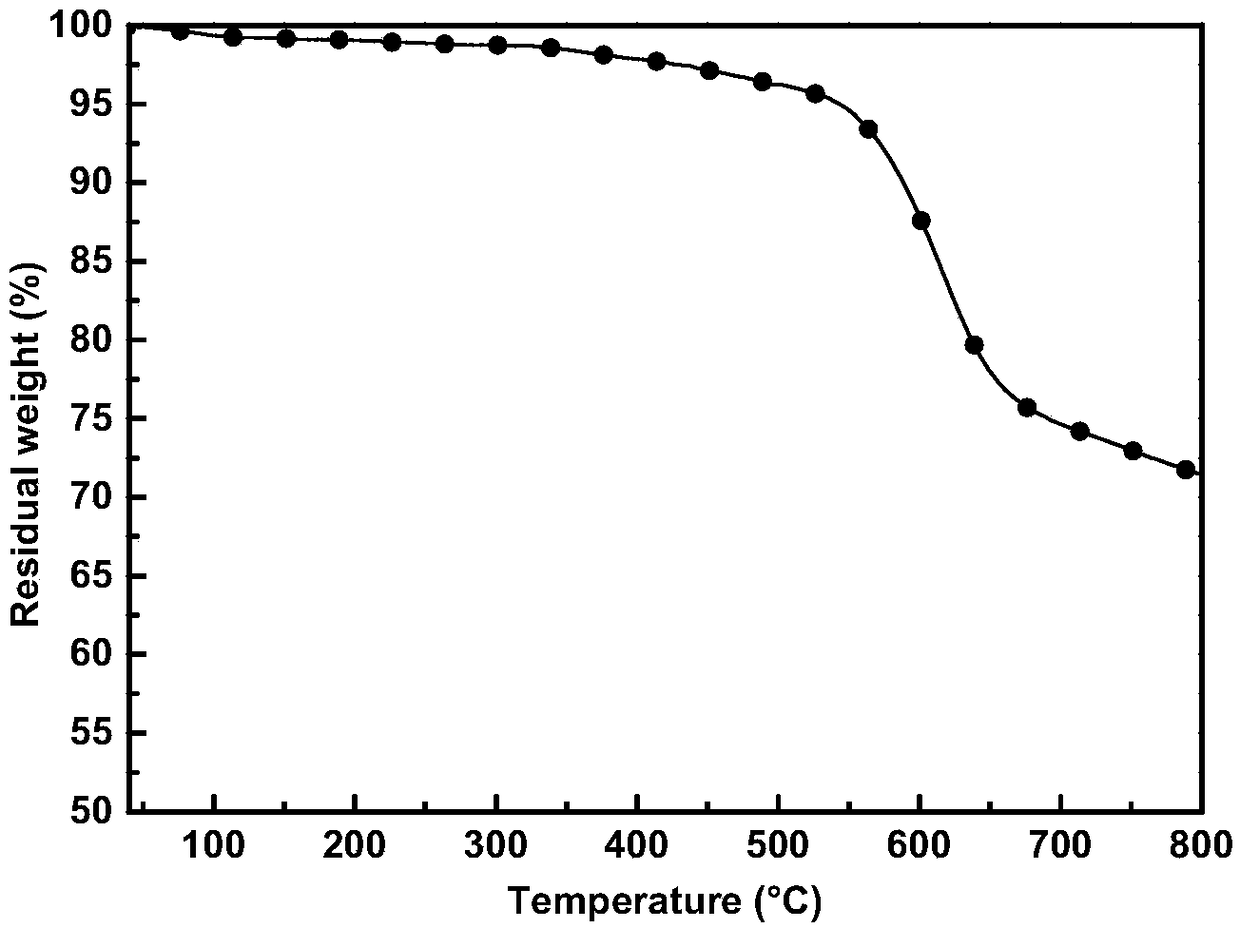
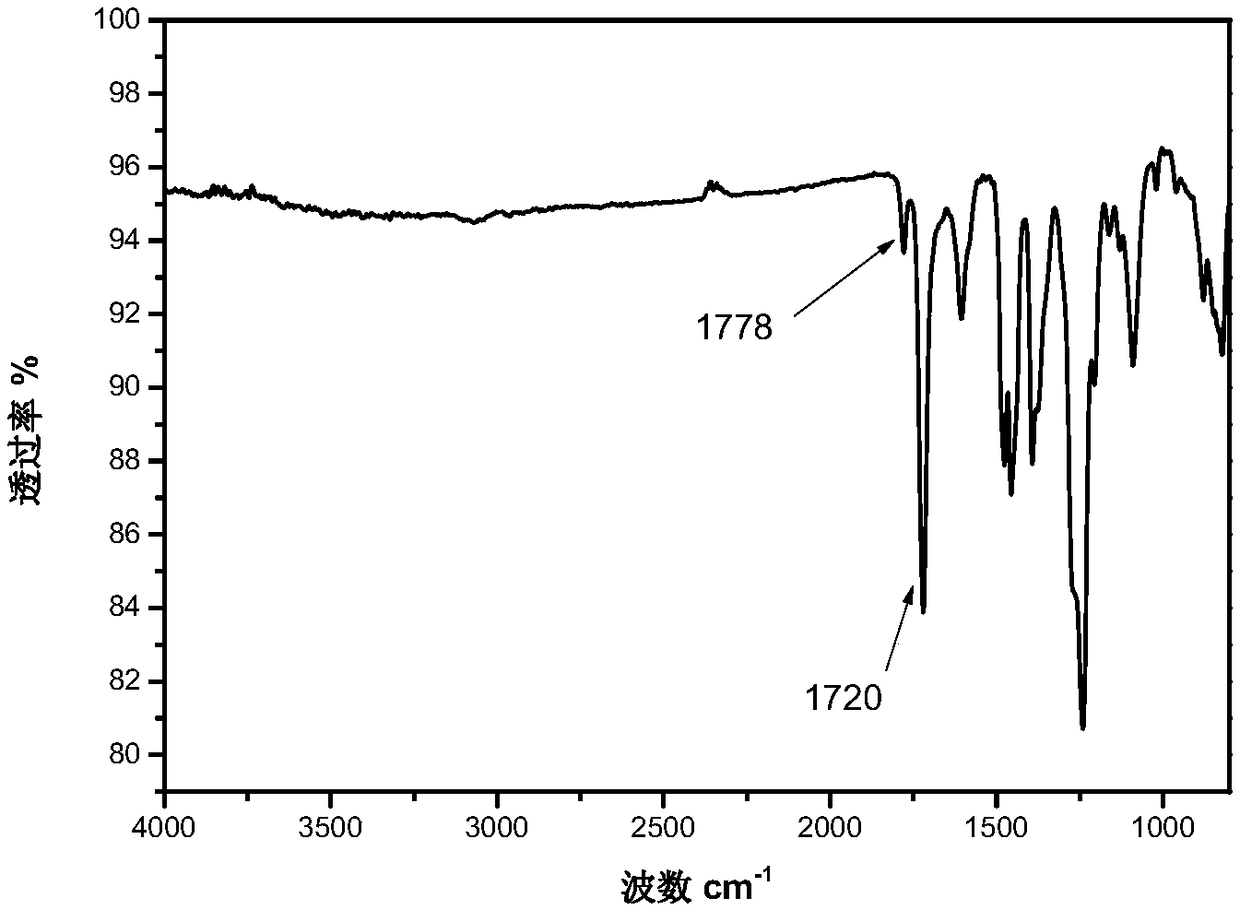
Diamine monomer with carbazole structure and polyimide synthesized by diamine monomer
A technology of diamine monomer and polyimide, applied in the field of polyimide, can solve problems such as single variety, and achieve the effect of easy operation, good application prospect and great application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] This example provides a synthesis method of 3,6-bis(4-aminophenyl)-9-phenylcarbazole and polyimide (1) 3,6-bis(4-aminophenyl)-9 -Synthesis of phenylcarbazole:
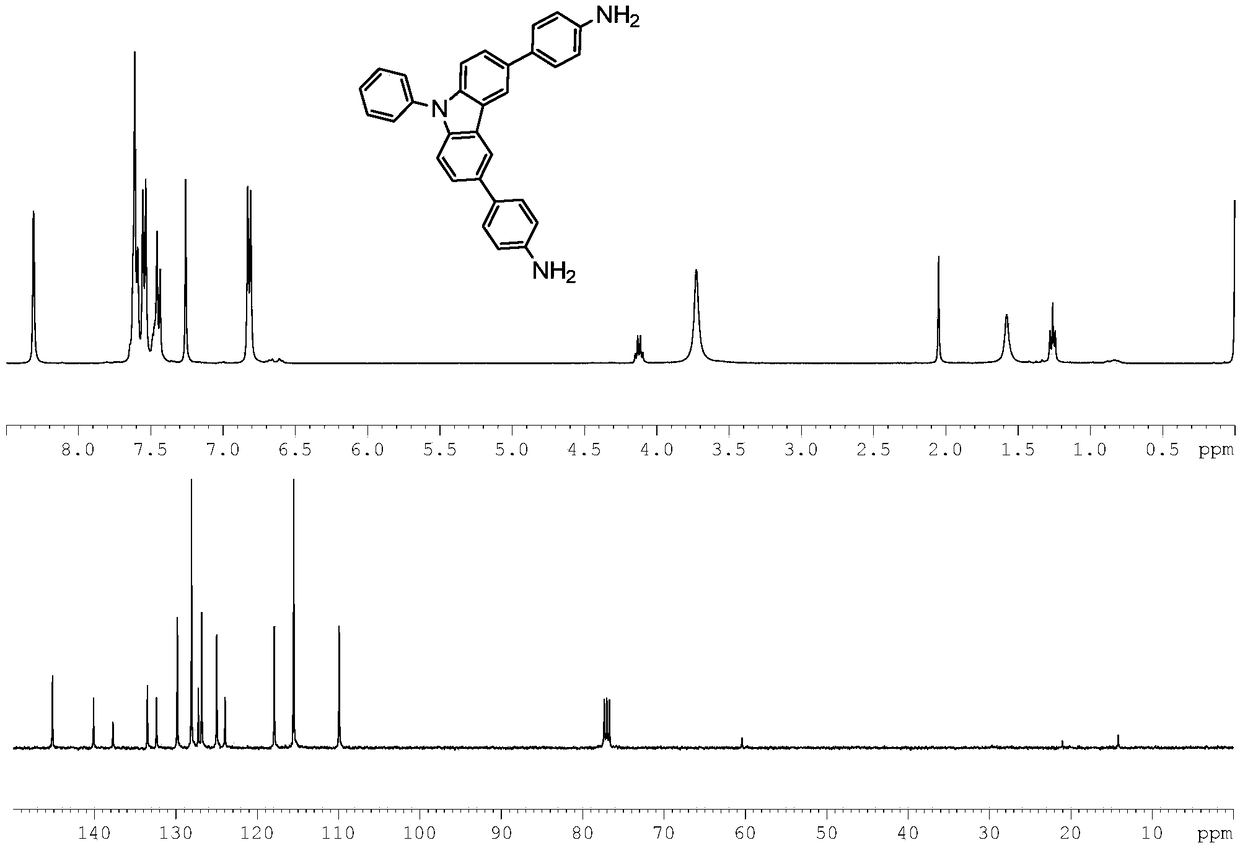
[0036] Dissolve 4.01g (10mmol) of 3,6-dibromo-9-phenylcarbazole and 3.47g (20mmol) of 4-aminophenylboronic acid hydrochloride into 30mL THF, then add 2mol / L K 2 CO 3 Solution 37.5mL, Aliquat 336 (phase transfer catalyst) 15 drops, under the protection of nitrogen, stir at room temperature for half an hour, then add tetrakis (triphenylphosphine) palladium catalyst to the system, react at 75°C for 24 hours. The reaction was detected by TLC. After the reaction was completed, the system was spin-dried, put on a silica gel column, and eluted with petroleum ether: ethyl acetate = 1:1 to obtain the product with a yield of 65%. product 1 H NMR and 13 C NMR spectrum as figure 1 As shown, it can be seen from the figure that the product synthesized by the reaction is 3,6-bis(4-aminophenyl)-9-phenylcarbazole of the pre...
Embodiment 2
[0041] This example provides a synthesis method of 3,6-bis(4-aminophenyl)-9-phenylcarbazole and polyimide (1) 3,6-bis(4-aminophenyl)-9 -Synthesis of phenylcarbazole:
[0042] Dissolve 8.02g (20mmol) of 3,6-dibromo-9-phenylcarbazole and 6.94g (40mmol) of 4-aminophenylboronic acid hydrochloride into 70mL THF, then add 2mol / L K 2 CO 3 Solution 75mL, Aliquat336 (phase transfer catalyst) 20 drops, under the protection of nitrogen, stir at room temperature for half an hour, then add tetrakis (triphenylphosphine) palladium catalyst to the system, react at 75°C for 24 hours. The reaction was detected by TLC. After the reaction was completed, the system was spin-dried, put on a silica gel column, and eluted with petroleum ether: ethyl acetate = 1:1 to obtain the product with a yield of 68%. 1 H NMR and 13 The characteristic peaks of the C NMR spectrum are the same as the characteristic peaks of the product detected in step (1) of Example 1, confirming that the product is 3,6-bis(4-a...
Embodiment 3
[0048] This example provides a synthesis method of 3,6-bis(4-aminophenyl)-9-phenylcarbazole and polyimide (1) 3,6-bis(4-aminophenyl)-9 -Synthesis of phenylcarbazole:
[0049] 16.04g (40mmol) of 3,6-dibromo-9-phenylcarbazole and 13.88g (80mmol) of 4-aminophenylboronic acid hydrochloride were dissolved in 150mL THF, followed by adding 2mol / L K 2 CO 3 Solution 180mL, Aliquat 336 (phase transfer catalyst) 40 drops, under the protection of nitrogen, stir at room temperature for half an hour, then add tetrakis (triphenylphosphine) palladium catalyst to the system, react at 85°C for 24 hours. The reaction was detected by TLC. After the reaction was completed, the system was spin-dried, put on a silica gel column, and eluted with petroleum ether: ethyl acetate = 1:1 to obtain the product with a yield of 70%. 1 H NMR and 13 The characteristic peaks of the C NMR spectrum are the same as the characteristic peaks of the product detected in step (1) of Example 1, confirming that the pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com