Human single chain variable fragments antibody for recognizing human c-Met proteins, diagnostic reagent and CAR-T cell preparation thereof
A single-source, antibody-based technology, applied in animal/human proteins, anti-animal/human immunoglobulins, cells modified by introducing foreign genetic material, etc., can solve the problems of poor specificity of cancer treatment techniques and achieve Huge social and economic benefits, significant specific effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1: Screening and verification of c-Met single chain antibody.
[0073] In Example 1, c-Met protein was used as an antigen to obtain a c-Met-specific single-chain antibody by screening a human single-chain antibody yeast display library.
[0074] Using prokaryotic expression vector, c-Met protein was produced by E.coli BL21DE3 as host cell. The complete c-Met protein contains an extracellular domain, a transmembrane domain and an intracellular domain.
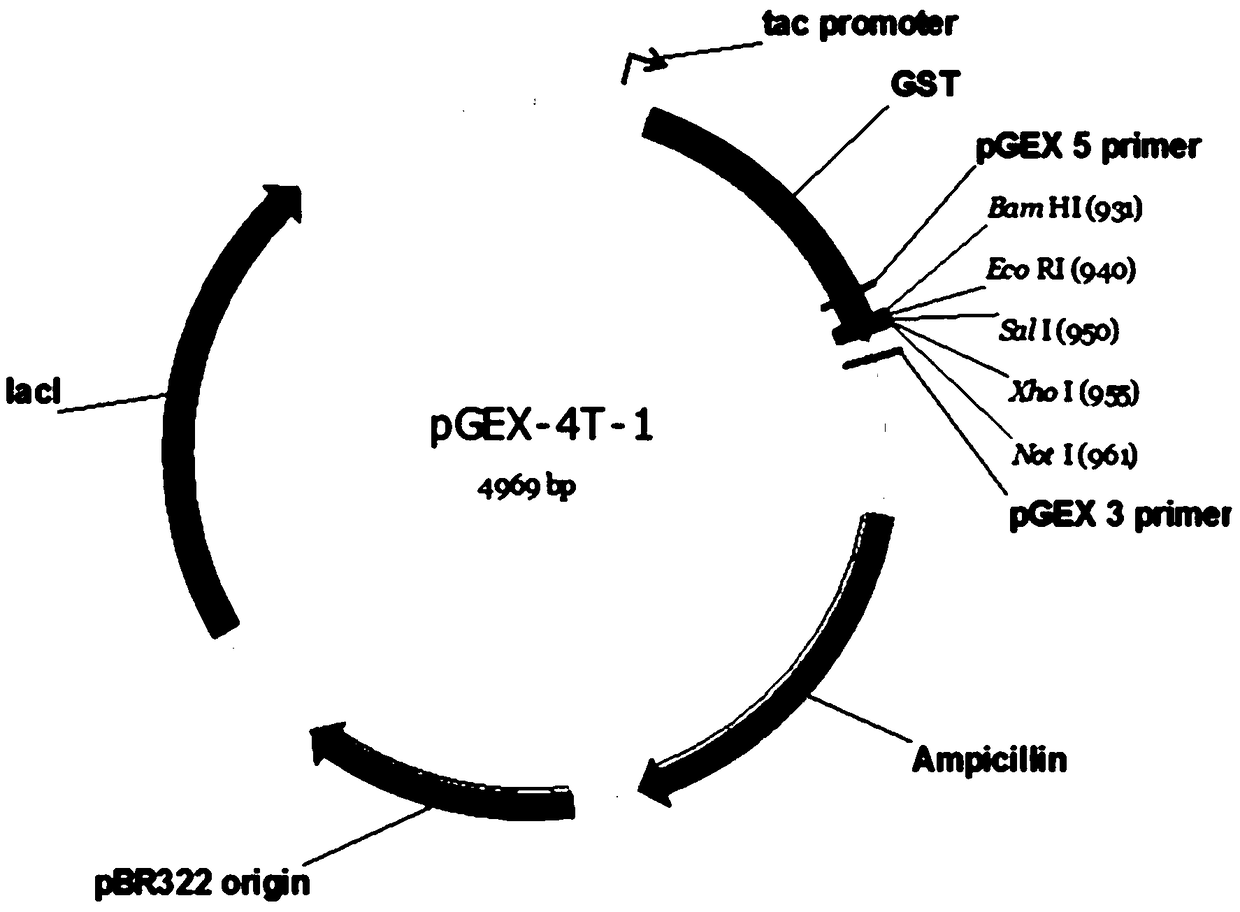
[0075] Extracellular region genes were amplified and cloned into e.g. figure 1 in the vector shown. This prokaryotic expression vector contains GST domain, so c-Met-GST fusion protein is expressed. Then, the fusion protein can be purified by GST purification column.
[0076] Such as figure 2 As shown, after SDS-PAGE gel electrophoresis of the purified protein, a single protein band was presented by Coomassie blue staining. The purified fusion protein was further labeled with biotin. Then, the labeled c-Met...
Embodiment 2
[0081] Example 2: Expression, purification and specificity verification of single chain antibody-Fc fusion protein.
[0082] In Example 2, the restriction endonucleases EcoRI and Kpn1 used for molecular cloning and the HiFiDNA assembly master mix used for vector construction were purchased from Neb Company (Massachusetts, USA); DNA gel recovery kit and plasmid extraction kit were purchased from Axygen Company (Shanghai, China); Competent cells Dh5α for vector construction were purchased from Quanshijin Biotechnology Co., Ltd. (Beijing, China); Pro293 for protein extraction TM CD Serum-free Medium was purchased from Lonza Company (U.S.); the protein G affinity chromatography purification column used for protein purification was purchased from GE Company (U.S.); the ultrafiltration tube used for protein concentration was purchased from Millipore Company (U.S.); MET antibody used for positive control was purchased from CST Company (USA); goat anti-mouse AlexaFluor594 was purchase...
Embodiment 3
[0100] Example 3: In vitro functional verification of single-chain antibody-Fc fusion protein.
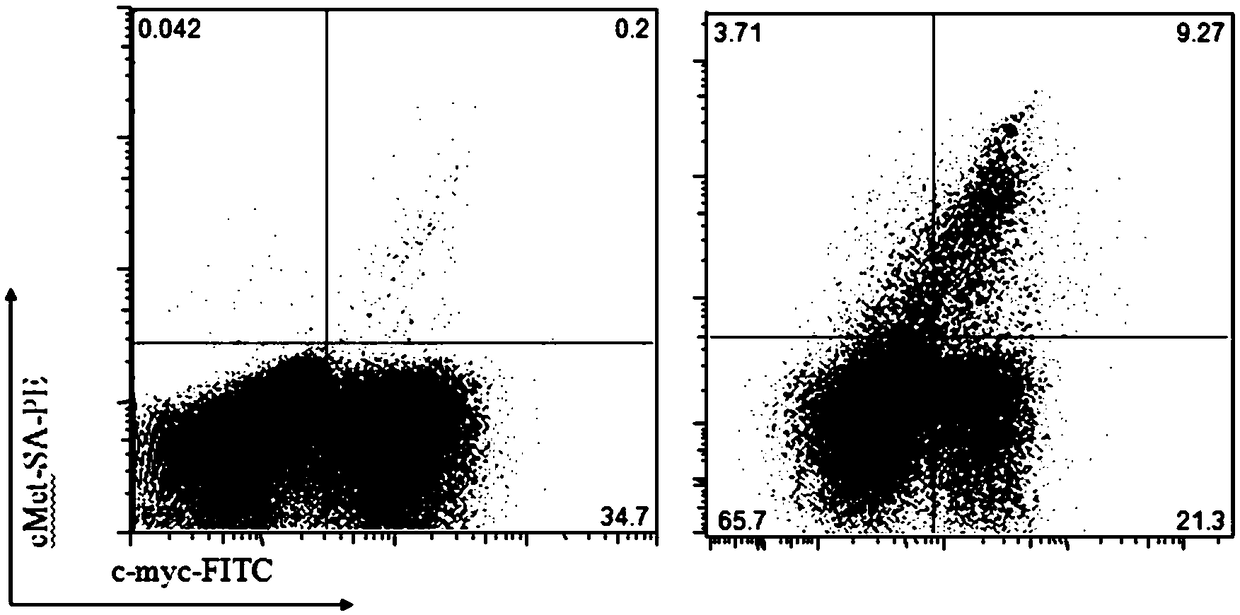
[0101] In Example 3, the functional verification of the fusion protein was further carried out, including using flow cytometry analysis and immunofluorescence to verify that the fusion protein specifically recognizes the c-Met protein expressed on the surface of tumor cells, and complement-dependent cytotoxicity, antibody-dependent Cytotoxicity test.
[0102] Among them, the process of flow cytometry experiment for detecting the specific recognition of c-Met by the single chain antibody-Fc fusion protein is as follows:
[0103] According to published literature, human lung adenocarcinoma A549 cells express c-Met protein. Commercialized anti-met antibody was incubated with A549 cells and combined with fluorescently labeled secondary antibody for flow staining. The results showed that there was migration of fluorescent signals, indicating that c-Met protein was expressed on the A54...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com