Non-steroidal anti-inflammatory eye drops and preparation method thereof
A technology of non-steroidal anti-inflammatory eye drops, which is applied in the field of medicine, can solve the problems of non-steroidal anti-inflammatory eye drops in terms of comfort and stability, and achieve significant economic benefits, strong targeting effect, and penetration powerful effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] The present invention also provides the preparation method of this non-steroidal anti-inflammatory eye drop, it is characterized in that comprising the steps:
[0025] A, disperse the flurbiprofen prodrug with an appropriate amount of water for injection, and set aside to obtain liquid A;
[0026] B. Disperse the thickener with an appropriate amount of water for injection and let it cool, then add the antibacterial agent, stir well and filter, add to the liquid A obtained in step A) to obtain liquid B;
[0027] C. Dissolve the pH adjuster with water for injection, slowly add it to liquid B, adjust the pH value to 5.5-7.5, and obtain liquid C;
[0028] D. Dissolve the osmotic pressure regulator with water for injection, slowly add to the solution C obtained in step 3), adjust the osmotic pressure molar concentration to 250-350mOsmol / kg, and obtain liquid D;
[0029] E. Add water for injection to the liquid D to make up the volume, so that the concentration of flurbiprof...
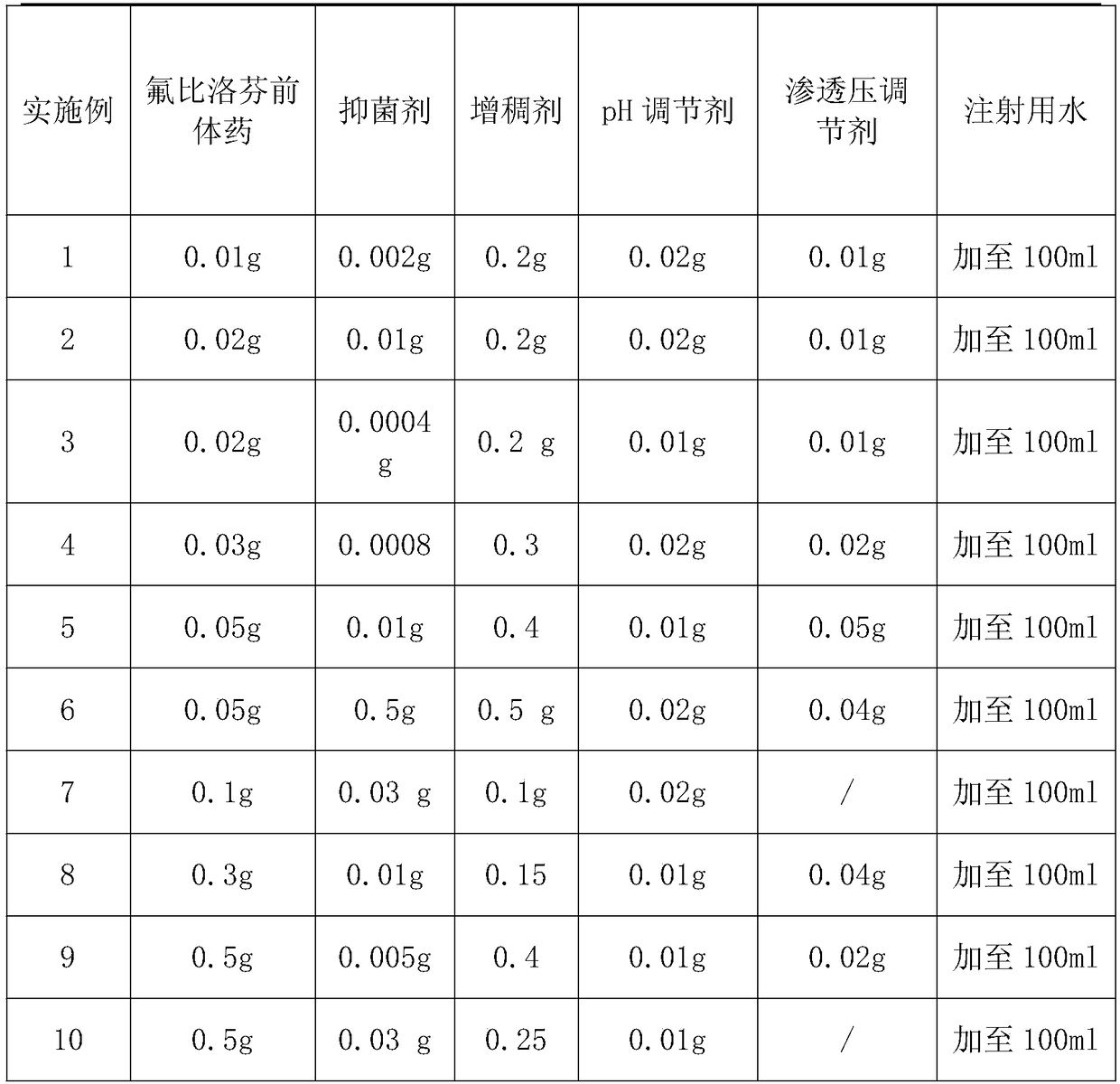
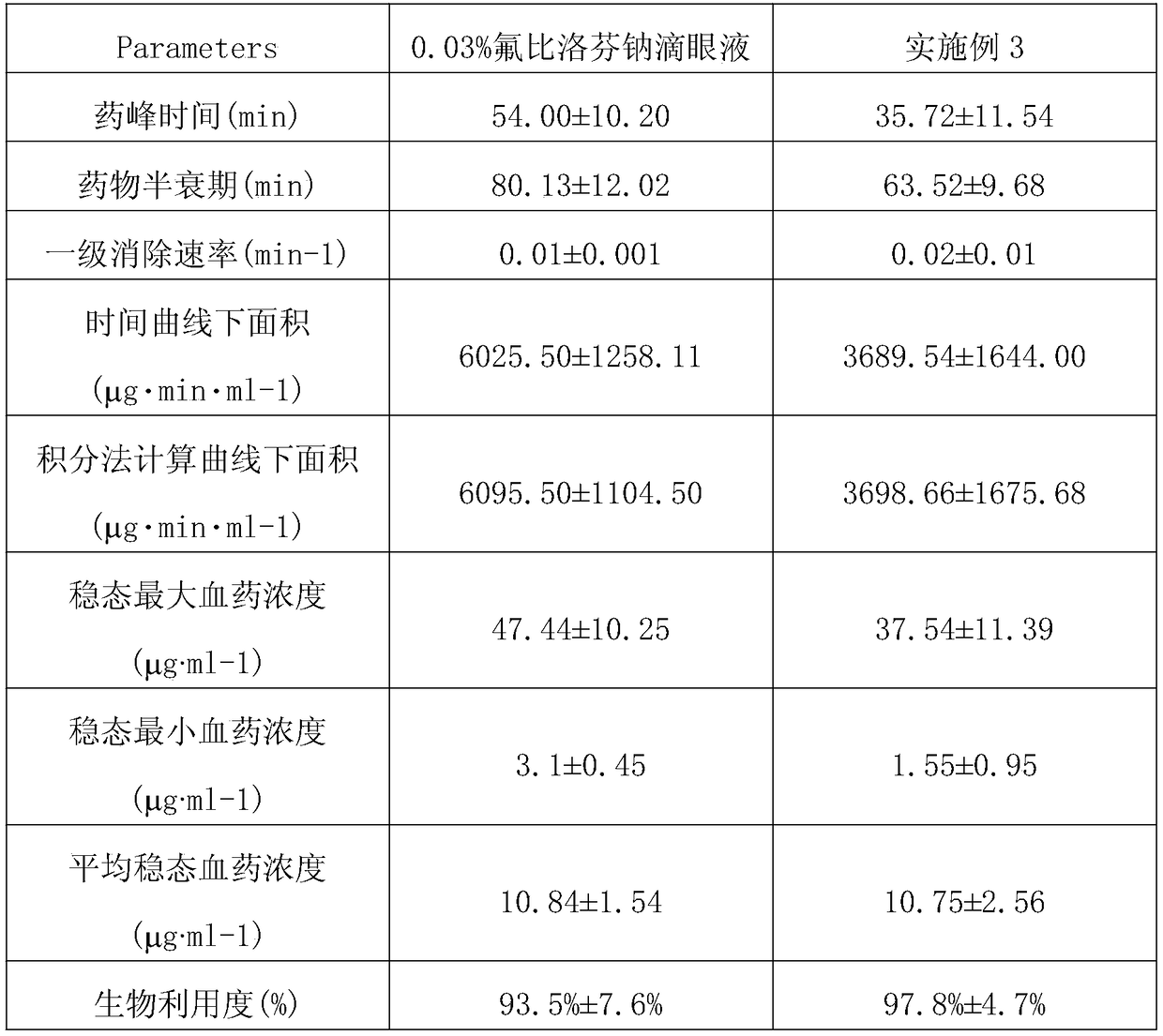
Embodiment 1-10
[0033] Raw materials and proportioning situation of table 1 eye drops
[0034]
Embodiment 1
[0036] At first will prepare raw materials by formula shown in table 1;
[0037] Step 1), dissolve the flurbiprofen prodrug with 50% water for injection, and set aside;
[0038] Step 2), disperse the thickener with water for injection and let cool, then add the antibacterial agent, stir and filter, and add to the flurbiprofen prodrug solution obtained in step 1);
[0039]Step 3), dissolve the pH regulator with water for injection, slowly add in the flurbiprofen prodrug solution obtained in step 2), adjust the pH value to 5.5 and stop;
[0040] Step 4), dissolve the osmotic pressure regulator with water for injection, slowly add to the solution obtained in step 3), adjust the osmotic pressure molar concentration to 250mOsmol / kg and stop adding;
[0041] Step 5), add water for injection to 100ml, filter, sub-package, to get final product;
[0042] Thimerosal is used as antibacterial agent, polyvinyl alcohol is used as thickener, sodium hydroxide solution and / or hydrochloric ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com