Chinese medicine effective part for treating depressive disorder and insomnia, preparation method and application thereof
A technology of effective parts and traditional Chinese medicines, which is applied to the effective parts of traditional Chinese medicines for treating depression and insomnia and the fields of preparation and application thereof, can solve the problems of clinical application limitation, respiratory inhibition, narrow antidepressant spectrum, etc., and achieves significant antidepressant and sedative effects. Hypnotic activity, good economic benefits and application prospects, the effect of improving appearance and solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Preparation method of SHF effective part
[0026] 1. The preparation method of effective part of SHF
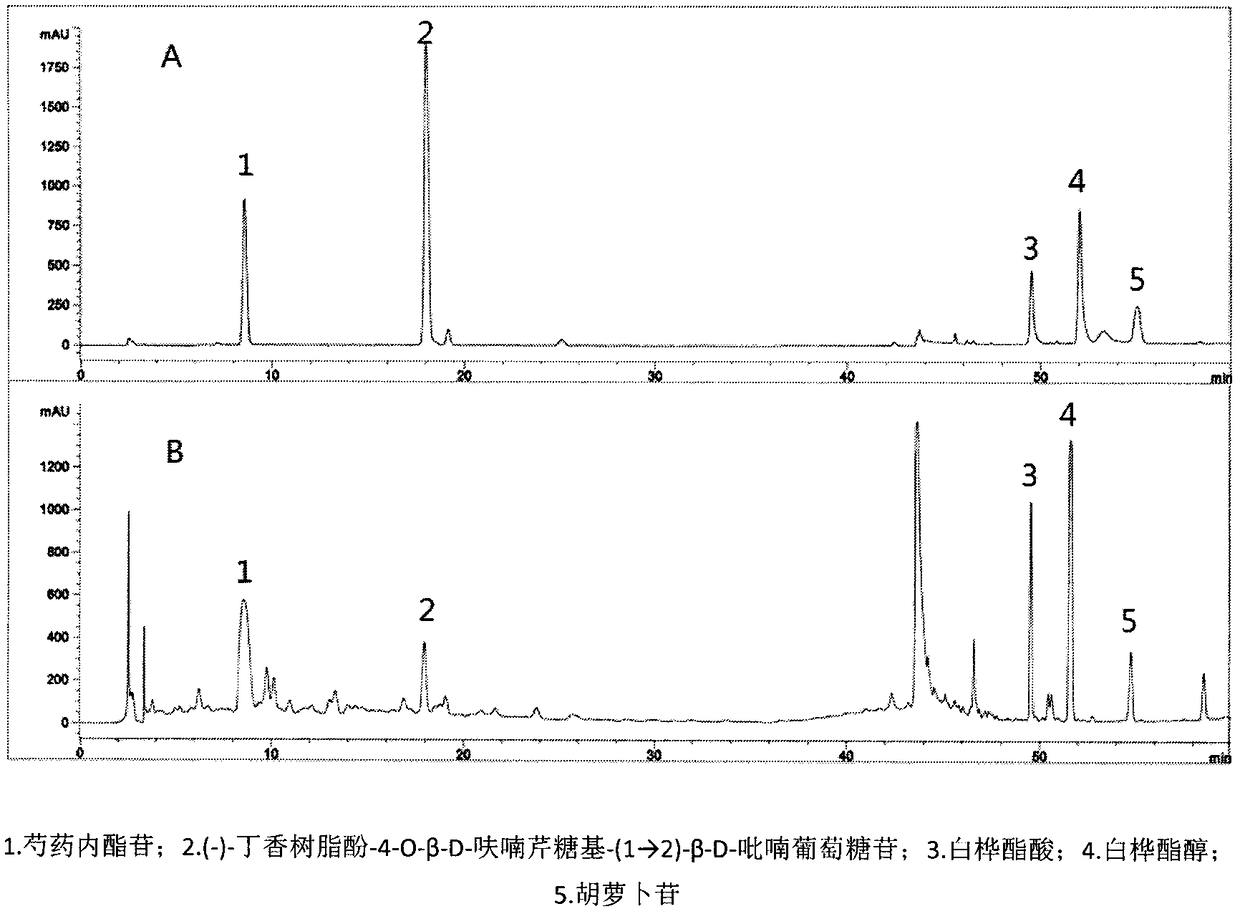
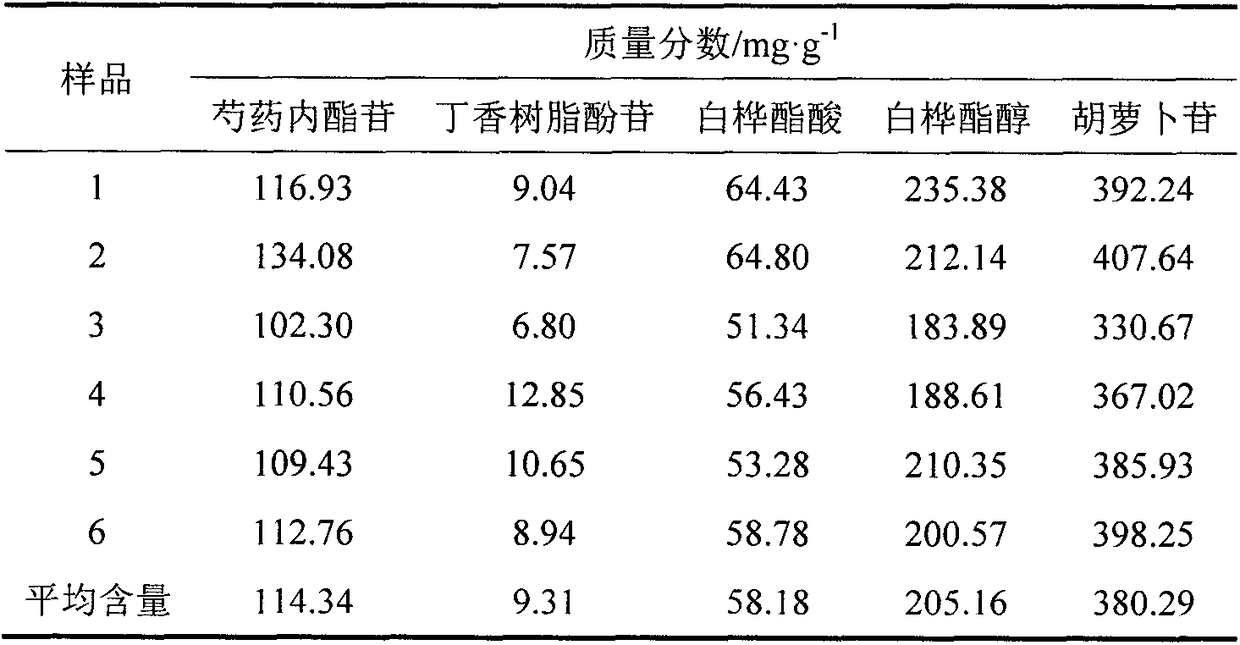
[0027] Weigh the four medicinal materials by weight (24g jujube seed, 13g bark of Albizia juniper, 6g white peony root, 10g cypress kernel), pulverize, heat and reflux with 10 times the amount and 8 times the amount of 70% ethanol for 2h and 1h respectively, filter, and combine The filtrate; the filtrate is concentrated under reduced pressure to the liquid extract, which is the compound SHF, for subsequent use; the liquid extract is dissolved in water, passed through the treated AB-8 macroporous adsorption resin, dissolved in distilled water respectively, passed through the treated AB-8 large Pore adsorption resin, eluted with 7BV 1% NaOH solution, 4BV distilled water, 5BV 70% ethanol solution respectively. The ethanol eluate was collected, concentrated under reduced pressure, and dried to obtain the effective fraction of SHF, wherein the percentage of total terpeno...
Embodiment 2
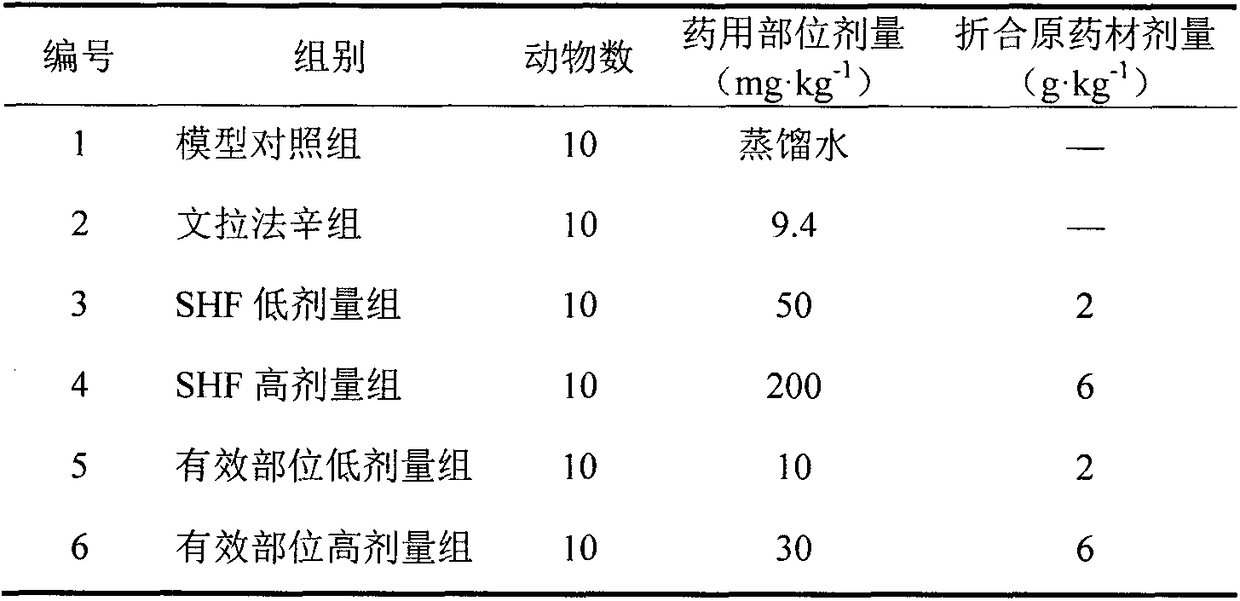
[0035] Experimental Study on Effective Parts of SHF in Treating Depression and Insomnia
[0036] 1. Experimental materials
[0037] 1.1 Experimental animals: 70 male ICR mice (clean grade, body weight 20±2g) from the Experimental Animal Center of the Institute of Radiological Medicine, Chinese Academy of Medical Sciences, certificate number: SCXKjin 2005-0001. Raised in the laboratory for 1 week before the experiment to adapt to the environment. The room temperature is 22±2°C, and the relative humidity is 65% to 70%. Sunlight for 12 hours, food and water ad libitum.
[0038] 1.2 Drugs and reagents: SHF extract, SHF active part extract (self-made in our laboratory); Venlafaxine hydrochloride sustained-release capsules (Effexor, Wyeth Pharmaceuticals, Ireland, batch number: 0902052).
[0039] 1.3 Experimental instruments: BP211D 1 / 100,000 analytical balance (Shanghai Junda Instrument Factory); R204 rotary evaporator (Shanghai Shensheng Technology Co., Ltd.); SHB-IIIA circulat...
Embodiment 4
[0101] Preparation of effective fraction granules
[0102] 1. Prescription screening of granules
[0103]1.1 Selection of excipients According to the prescription designed in Table 7, take quantitative effective part dry cream powder and excipients (through 80 mesh sieve) and pass through 40 mesh sieve, mix evenly, use 70% ethanol as binder to make soft material, pass through 20 mesh sieve to granulate, 60U Blow drying for 20 minutes, pass through a 18-mesh sieve for granulation, and dry at 60°C for 10mm. Calculate the fine powder rate, see Table 7.
[0104] 1.2 Determination of Solubility Precisely weigh 1.0g of each of the three granules under Item 1.1, add 200mL of water at about 60°C, shake, and measure the time required for complete dissolution. The appendix of the 2010 edition of the Chinese Pharmacopoeia stipulates that the granules should be completely dissolved within 5 minutes, and slight turbidity is allowed. Results The three granules were all dissolved in 2 minu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com