Design, synthesis and application of tumor-targeted near infrared fluorescence imaging agent
A fluorescent imaging agent and tumor-targeting technology, applied in the field of precise resection surgery, can solve the problem of low tumor specificity and achieve good specificity and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
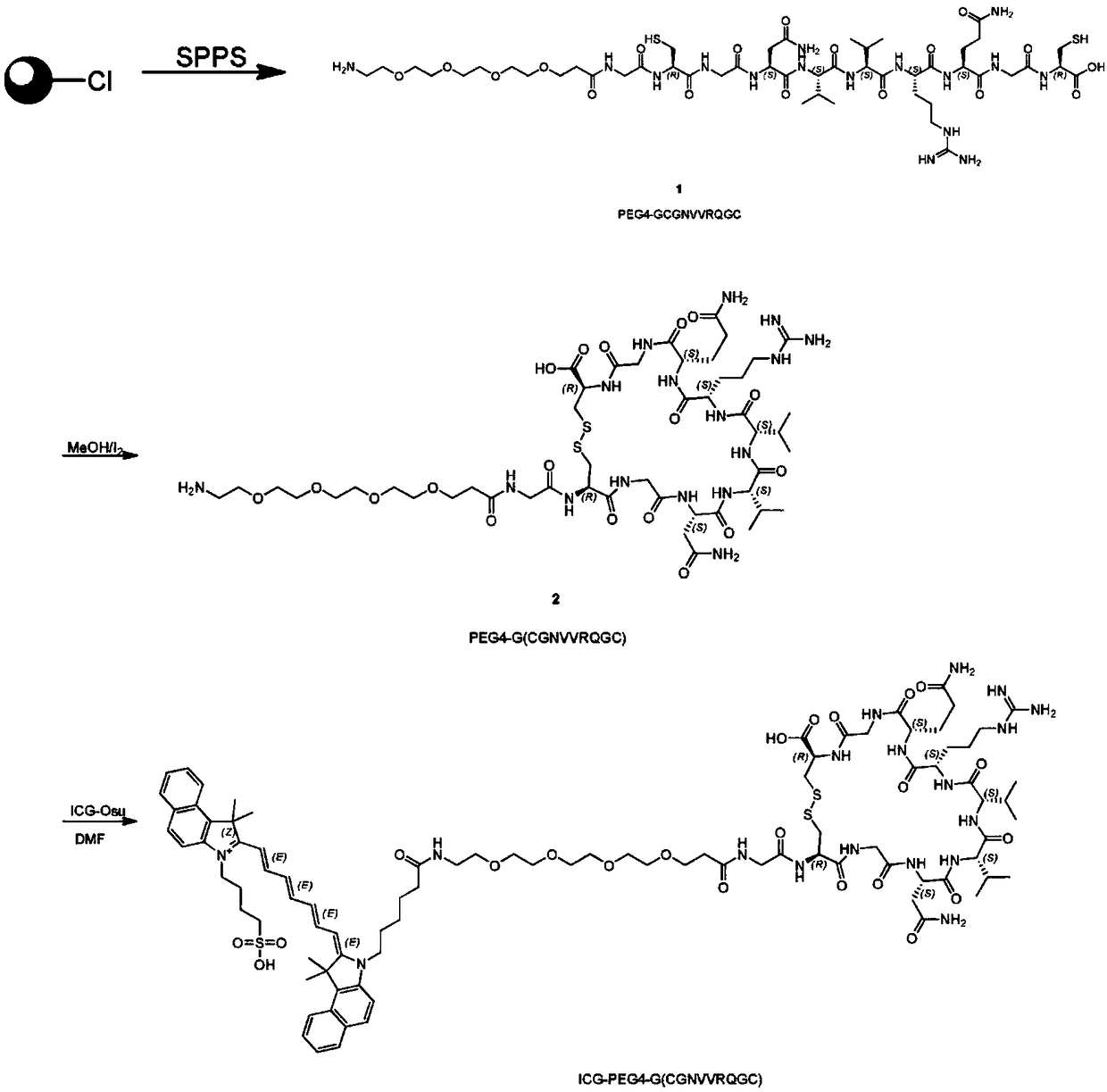
[0030] Example 1 Preparation of ICG-OSu-PEG4-TMTP1
[0031] see figure 1 , the preparation process of ICG-OSu-PEG4-TMTP1 is as follows: 1) use the solid phase carrier to synthesize the polypeptide GCGNVVRQGC according to the designed amino acid sequence, and covalently link it to PEG4; 2) remove the polypeptide from the resin, and use MEOH / I 2 Oxidation, the polypeptide sequence is bridged by a disulfide bond to form a ring, that is, PEG4-G (CGNVVRQGC), and then separated, purified, and freeze-dried; 3) The product obtained in step 2) is mixed with ICG-Osu at a ratio of 1:1 , dissolved in DMF, then added 2 times the volume of DIEA, and reacted for 10 minutes. After the reaction was identified by LCMS, it was separated, purified, and freeze-dried to obtain the imaging agent ICG-OSu-PEG4-TMTP1 of the present invention. The synthesis process was entrusted to Shanghai WuXi AppTec New Drug Development Co., Ltd.
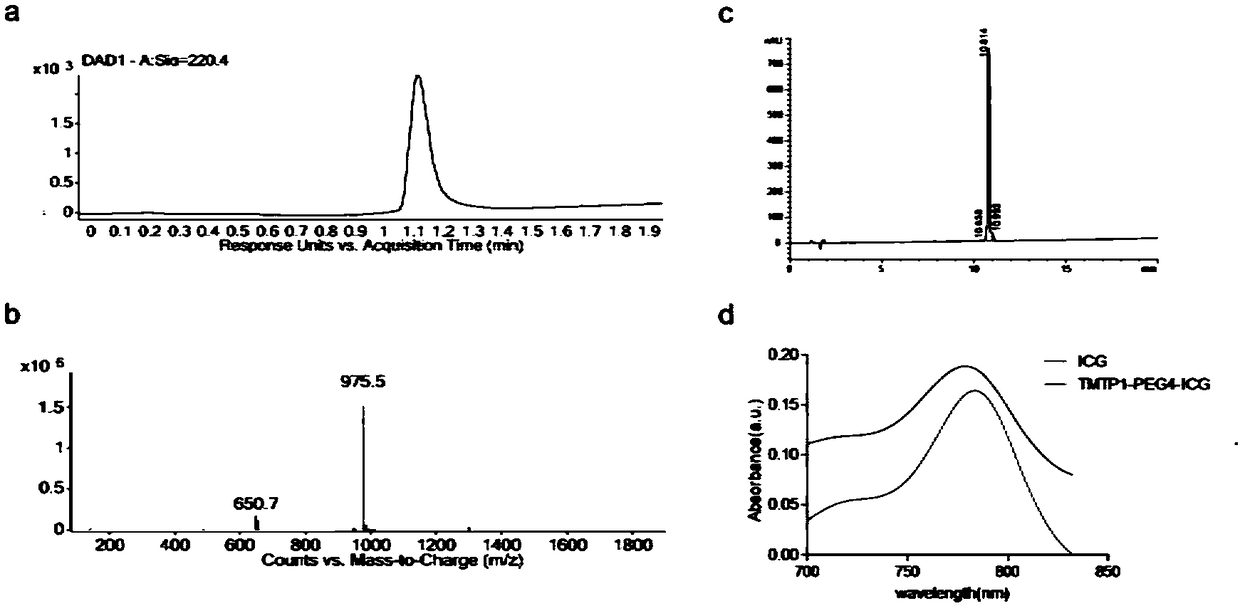
[0032] see figure 2 , figure 2 a and 2b are detected by liquid ...
Embodiment 2
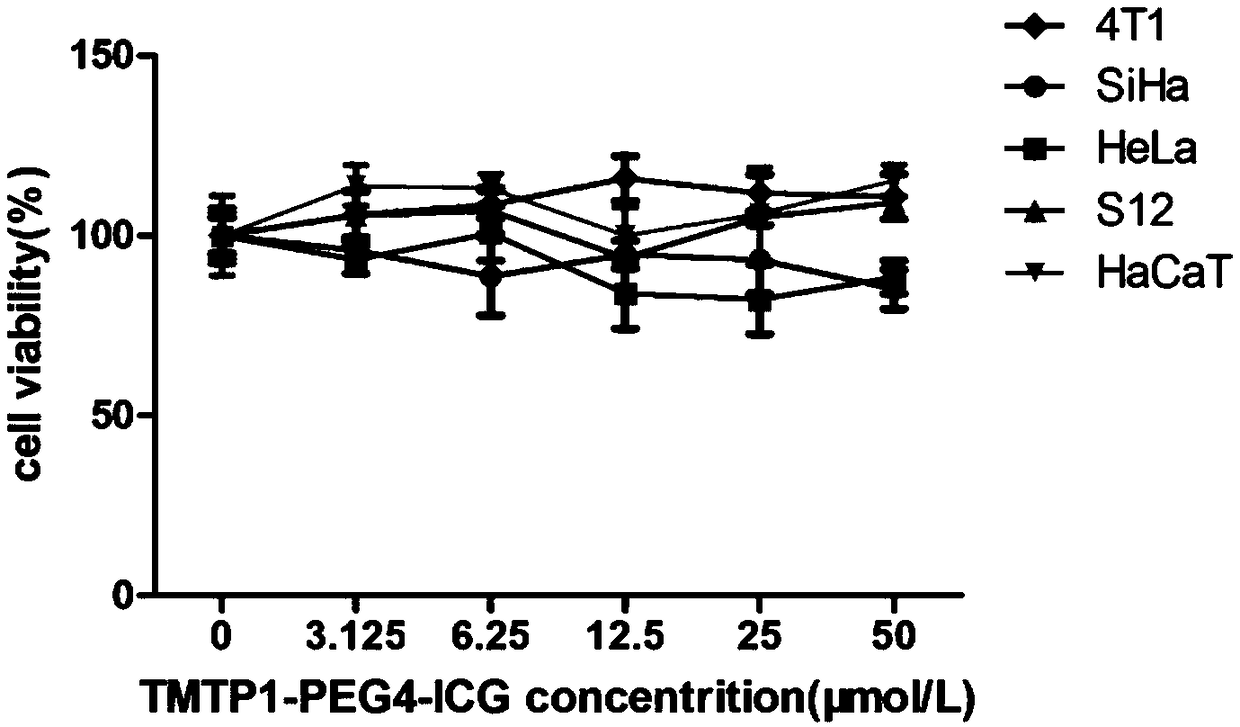
[0033] Embodiment 2 Cytotoxicity test:
[0034] 1. Cell Culture
[0035] The mouse breast cancer cell line 4T1 (purchased from ATCC cell bank in the United States) was cultured with RPMI-1640 medium (Gibco, ThermoFish Company, USA) containing 10% fetal bovine serum by volume fraction, and the cervical cancer cell lines HeLa and SiHa (purchased from the U.S. ATCC cell bank) and normal skin keratinized epithelial cells (purchased from China CCTCC cell bank) were cultured in DMEM medium (Gibco, ThermoFish Company, USA) containing 10% fetal bovine serum by volume fraction, and cervical intraepithelial neoplasia cell line S12 (Gifted by Professor Kenneth Raj, UK) with a volume fraction of 5% fetal bovine serum, 5 μg / ml insulin (Sigma-Aldrich, USA), 8.4ng / ml cholera toxin (Sigma-Aldrich, USA), 24.3 μg / ml glandular Purine (Sigma-Aldrich, USA), 0.5 μg / ml hydrocortisone (Sigma-Aldrich, USA) and 10 ng / ml endothelial factor (EGF, Peprotech) in F12 and DF12 medium (Gibco, ThermoFish Comp...
Embodiment 3
[0039] Example 3 Experiment of tumor cell binding efficiency under different PEG numbers
[0040] The imaging agents with different numbers of PEG were synthesized according to the method shown in Example 1, taking n=2, 4, 6, 8, 10, 20 respectively. Cultivate 4T1 cells and HeLa cells as shown in Example 2, and 4T1 and HeLa cells were prepared according to the ratio of 1*10 5 Plant a 24-well plate at a density of one per well. After overnight incubation, add 500ul of 1μM ICG and ICG-OSu-(PEG)n-TMTP1 to each well, and add PBS to the well as a blank control. After incubation on ice for 20 minutes, use Wash with cold PBS 3 times, and place under a small animal in vivo imager to collect fluorescence pictures of the well plate. Then, the fluorescence value of each well was quantitatively analyzed by the ROI tool of the IVIS imaging software.
[0041] For experimental results, see Figure 4 , Figure 4 a is the fluorescence image of 4T1 cells incubated with different PEG numbers ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com