a tio 2 -mofs photocatalyst and its preparation method and application
A technology of photocatalysts and photocatalytic materials, applied in the direction of catalyst activation/preparation, physical/chemical process catalysts, organic compounds/hydrides/coordination complex catalysts, etc., can solve the problems of low activity, low mineralization rate, easy Carbon deposition and deactivation problems, to achieve the effect of simple operation, short cycle, rich pore structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
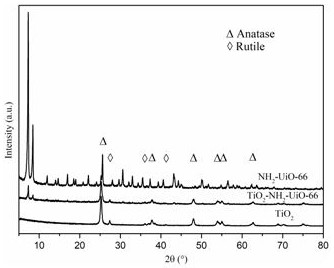
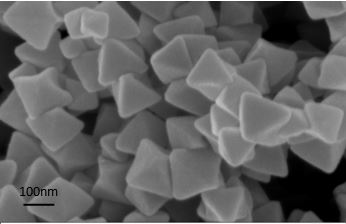
[0031] NH 2- Preparation of UiO-66: Add 0.05 g of zirconium tetrachloride and 0.08 g of 2-aminoterephthalic acid to 60 mL of dimethylformamide to obtain a mixed solution A, stir at 25 °C at 25 r / min for 10 min, then add 2 mL of glacial acetic acid solution to the mixed solution A, mix and stir for 10 min to obtain the mixed solution B, transfer the mixed solution B to the liner of the polytetrafluoroethylene reactor, and then put the polytetrafluoroethylene reactor The liner was placed in a high-pressure reactor, and hydrothermally reacted at 120 °C 0.18 Mpa for 24 h. After natural cooling, the precipitate was washed with dimethylformamide and methanol respectively, soaked in methanol and vacuum-dried at 100 °C for 12 h, and finally obtained NH 2 -UiO-66.
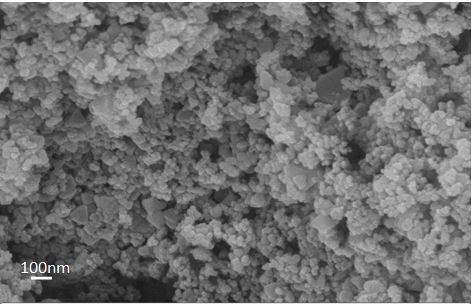
[0032] TiO 2 -NH 2 -Preparation of UiO-66: 0.25 gTiO 2 Added into 50 mL of methanol to obtain a mixed solution C, stirred at room temperature for 10 min, and then 0.75 g of NH 2 -UiO-66 was added to mixed solution C, ...
Embodiment 2
[0034] NH 2 - Preparation of UiO-66: Add 0.06 g of zirconium tetrachloride and 0.09 g of 2-aminoterephthalic acid to 50 mL of dimethylformamide to obtain a mixed solution A, stir at 30 °C at 25 r / min for 15 min, then add 4 mL of glacial acetic acid solution to the mixed solution A, mix and stir for 15 min to obtain the mixed solution B, transfer the mixed solution B to the liner of the polytetrafluoroethylene reactor, and then put the polytetrafluoroethylene reactor The liner was placed in a high-pressure reactor, and subjected to hydrothermal reaction at 120 °C 0.20 Mpa for 24 h, and then the solution was washed with dimethylformamide and methanol respectively, soaked in methanol and vacuum-dried at 110 °C for 10 h to finally obtain NH 2 -UiO-66.
[0035] TiO 2 -NH 2 -Preparation of UiO-66: 0.50 g TiO 2 Added into 50 mL of methanol to obtain a mixed solution C, stirred at room temperature for 10 min, and then 0.50 g of NH 2 -UiO-66 was added to mixed solution C, mixed an...
Embodiment 3
[0037] NH 2 - Preparation of UiO-66: Add 0.06 g of zirconium tetrachloride and 0.09 g of 2-aminoterephthalic acid to 60 mL of dimethylformamide to obtain a mixed solution A, stir at 26 °C at 25 r / min for 15 min, then add 4 mL of glacial acetic acid solution to the mixed solution A, mix and stir for 15 min to obtain the mixed solution B, transfer the mixed solution B to the liner of the polytetrafluoroethylene reactor, and then put the polytetrafluoroethylene reactor The liner was placed in a high-pressure reactor, and subjected to hydrothermal reaction at 120 °C 0.17 Mpa for 24 h, then the solution was washed with dimethylformamide and methanol respectively, soaked in methanol and vacuum-dried at 110 °C for 10 h, and finally NH 2 -UiO-66.
[0038] TiO 2 -NH 2 -Preparation of UiO-66: 1 g TiO 2 Added into 50 mL of methanol to obtain a mixed solution C, stirred at room temperature for 10 min, and then 0 g NH 2 -UiO-66 was added to the mixed solution C, mixed and stirred for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com