Compound as well as synthesis method and application thereof
A compound and manufacturing method technology, applied in the directions of active ingredients of heterocyclic compounds, chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, etc. lower problem
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
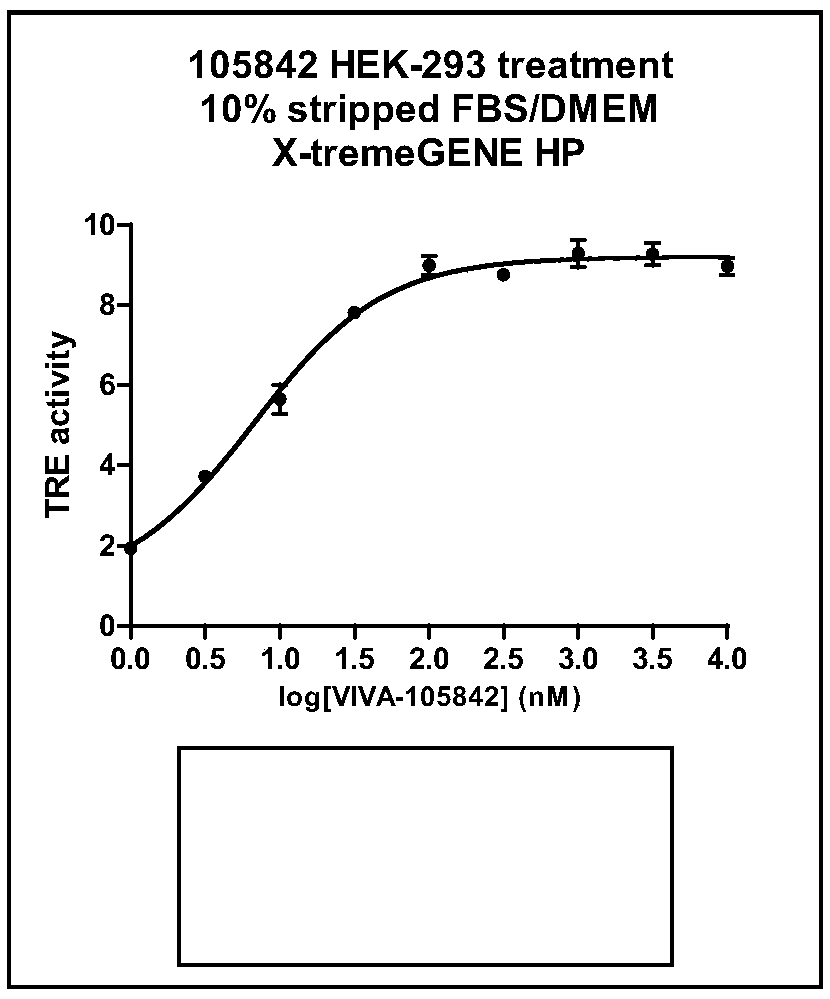
Image
Examples
Embodiment 1
[0101] Embodiment 1, product number TDM-105842
[0102] 2-(3,5-dichloro-4-(3-(4-methyl-1H-pyrazole-2-methyl)-4-hydroxyphenoxy)phenyl)-1,2,4-tri Oxazine-3,5(2H,4H)-dione
[0103] Structural formula:
[0104]
[0105] synthetic route:
[0106]
[0107] Specific synthesis method:
[0108] Step 1: Synthesis of 1,3-dichloro-2-(4-methoxyphenoxy)-5-nitrobenzene
[0109]
[0110] Dissolve 4-methoxyphenol (5.5 g, 44 mmol) in DMF (100 mL) and add NaH (2.64 g, 66 mmol), stir for half an hour and add 1,2,3-trichloro-5-nitrobenzene (10 g, 44mmol). The reaction solution was heated to 120°C for three hours. The reaction was completed and cooled to room temperature, then concentrated. The residue was quenched with water and extracted with ethyl acetate. The organic layer was dried and concentrated to obtain the crude product, and then column chromatography (0-10% ethyl acetate and petroleum ether) gave the product (5.0 g, 36%).
[0111] In this step, different reaction condi...
Embodiment 2
[0160] 2-(3,5-dichloro-4-(3-(4,4'-difluoro-cyclohexyl-2-methyl)-4-hydroxyphenoxy)phenyl)-1,2,4- Triazine-3,5(2H,4H)-dione
[0161] Structural formula:
[0162]
[0163] m / z 499[M+H] +
Embodiment 3
[0165] 2-(3,5-dichloro-4-(3-(1P-cyclohexyl-2-methyl)-4-hydroxyphenoxy)phenyl)-1,2,4-triazine-3,5 (2H,4H)-Diketone
[0166] Structural formula:
[0167]
[0168] m / z 481[M+H]+
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com