Separation method of two-phase structure in duplex stainless steel, reticular ferrite microporous material
A technology of duplex stainless steel and separation method, which is applied in the field of stainless steel, can solve problems such as the inability to obtain a single organizational structure, and achieve the effect of wide application range and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
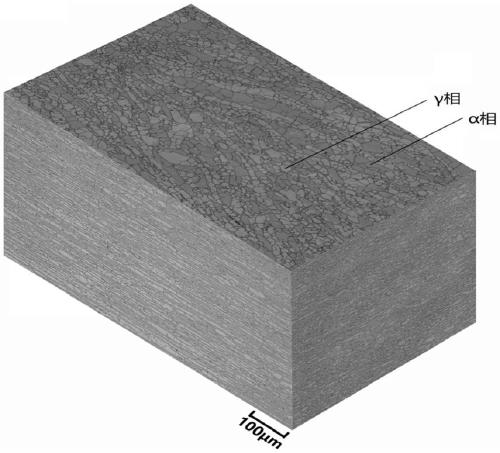
[0064] 2507 duplex stainless steel was used for testing. The chemical composition weight percent of the steel is as follows: C 0.020, Mn0.75, P 0.026, S 0.002, Si 0.21, Ni 6.82, Cr 25.04, Mo 3.56, N 0.26, and the rest are Fe and unavoidable impurity elements. The structure of the 2507 plate obtained by hot rolling and solution heat treatment at 1050°C for 1h, such as image 3 As shown, the proportion of ferrite α phase is about 42% (volume percentage):
[0065] Prepare a mixed solution of 2.0M copper sulfate (volume molar concentration) + 1.0M hydrochloric acid (volume molar concentration), and place it in a 700mL electrochemical cell, which is heated to 50±0.5°C with a water bath.
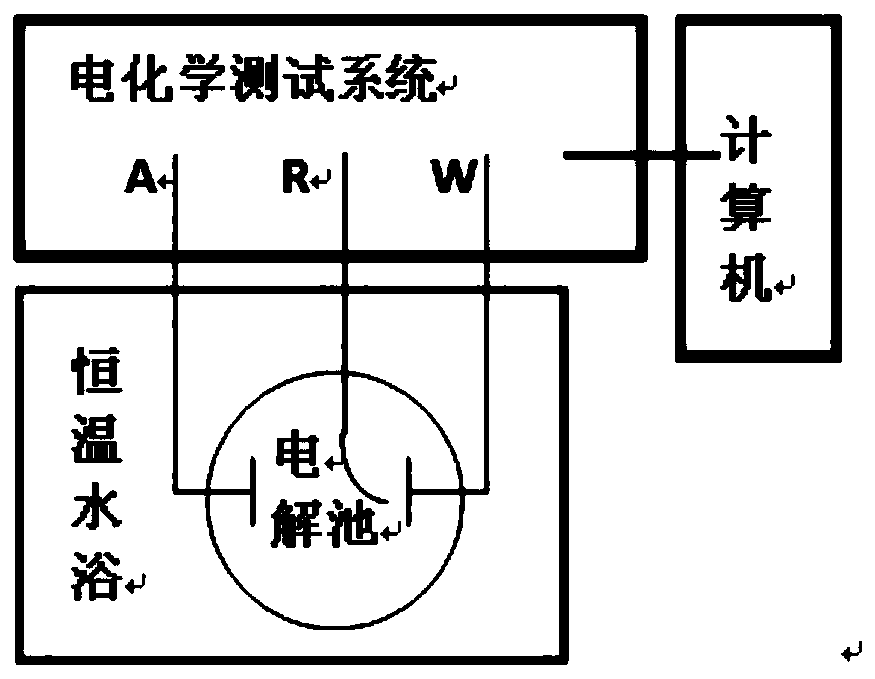
[0066] A sample of 10×10×3.5mm (length×width×thickness) was cut from the plate, and the surface was machined into a six-sided pattern to prepare an electrochemical sample. The sample was used as the working electrode W, saturated with calomel The electrode is used as the reference electrode R, a...
Embodiment 2
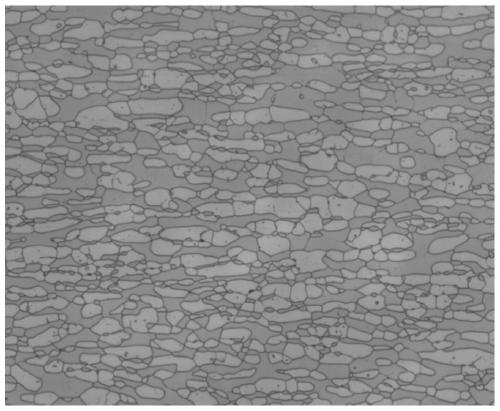
[0070] 2101 duplex stainless steel was used for testing. The chemical composition weight percent of the steel is as follows: C 0.028, Mn 4.58, P0.022, S 0.0043, Si 0.6, Ni 1.99, Cr 20.98, Mo 0.03, N 0.22, and the rest are Fe and unavoidable impurity elements. The structure of the 2101 plate obtained by hot rolling and solution heat treatment at 1080°C for 3 hours, such as Figure 7 As shown, the proportion of ferrite α phase is about 67% (volume percentage):
[0071] Prepare a mixed solution of 0.3M copper sulfate (volume molar concentration) + 2.5M hydrochloric acid (volume molar concentration), and place it in a 700mL electrochemical cell, which is heated to 50±0.5°C with a water bath.
[0072] Cut and grind a sample of 10×10×0.5mm (length×width×thickness) from the plate, prepare an electrochemical sample, use the sample as the working electrode W, and use the saturated calomel electrode as the reference electrode R, platinum is used as the auxiliary electrode A, and a thr...
Embodiment 3
[0075] 2205 duplex stainless steel was used for testing. The chemical composition weight percent of the steel is as follows: C 0.025, Mn 1.18, P0.022, S 0.003, Si 0.56, Ni 4.86, Cr 22.41, Mo 3.16, N 0.14, and the rest are Fe and unavoidable impurity elements. The structure of the 2205 plate obtained by hot rolling and solution heat treatment at 1080 ° C for 1 h, such as Figure 10 As shown, the proportion of ferrite α phase is about 55% (volume percentage):
[0076] Prepare a mixed solution of 2.0M copper sulfate (volume molar concentration) + 2.0M hydrochloric acid (volume molar concentration), and place it in an 800mL electrochemical electrolytic cell, which is heated to 50±0.5°C with a water bath.
[0077] Cut a sample of 30×30×5mm (length×width×thickness) from the plate, machine the surface into a six-sided pattern, prepare an electrochemical sample, and use the sample as a working electrode W, a saturated calomel electrode As the reference electrode R and platinum as th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com