A kind of bacterial polysaccharide conjugated vaccine and its preparation method and application
A technology of combining bacterial polysaccharides and vaccines, applied in the field of biomedicine, to achieve the effects of increasing the content of free polysaccharides, enhancing specific antibody titers, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] 1. Bacterial polysaccharide crushing:
[0078] The original polysaccharide of meningitis capsular polysaccharide (CPS) has a large molecular weight and high viscosity, and it is easy to produce macromolecular cross-linking and reduce the stability. The invention adopts ultrasonic waves to crush polysaccharides into polysaccharides with uniform molecular weight. In the ultrasonic wave, the polysaccharide produces rapid mechanical movement, and the molecules are degraded into smaller molecules with the fluctuating high-speed vibration and shear force in the medium, and have no effect on the polysaccharide structural unit. The specific methods and steps are as follows:
[0079] (1) Weigh a certain amount of group C meningococcal capsular polysaccharide (CPS), and dilute it with 20mmol / L sodium phosphate buffer containing 0.15mol / L sodium chloride at pH=7.2, so that the final concentration of polysaccharide is 2mg / L mLl;
[0080] (2) Use No. 3 probe for ultrasonic crushi...
Embodiment 2
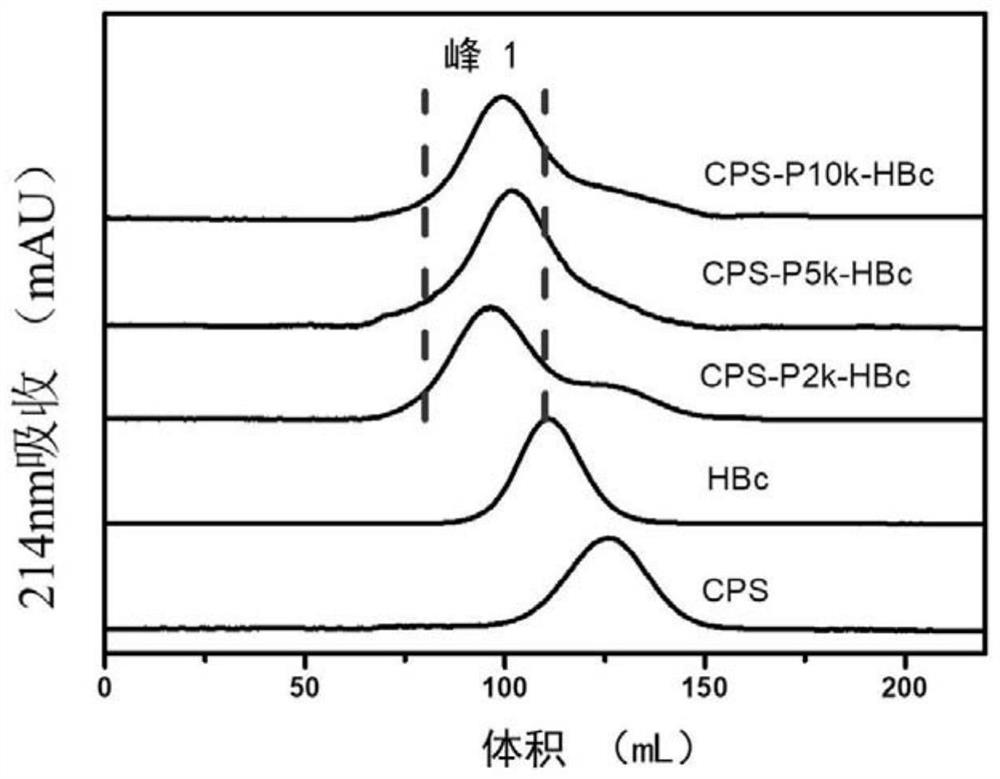
[0099] Characterization of Bacterial Polysaccharide Conjugate Vaccine
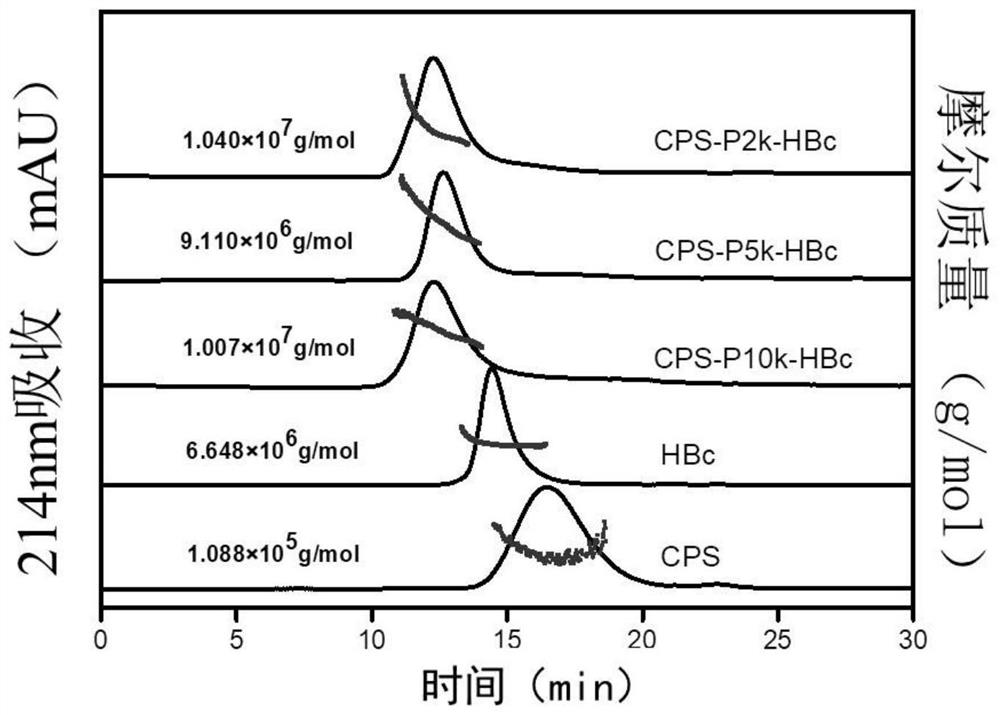
[0100] Molecular weight determination of polysaccharide-conjugated vaccines by multi-angle laser light scattering. The dn / dc value of HBc virus-like particles was 0.185mL / g, the dn / dc value of polysaccharide was 0.171mL / g, and the molecular weights of unbound HBc and CPS were 6.64×103kDa and 1.08×102kDa respectively by multi-angle laser scattering analysis. Compared with the unconjugated polysaccharide protein, the retention time of the three conjugated vaccines was significantly reduced, indicating that the polysaccharide protein conjugation was successful. In order to calculate the molecular weights of the three conjugated vaccines, the refractive index increments dn / dc of the three conjugated vaccines were calculated by the following formula I.
[0101]
[0102] R in the formula represents the ratio of polysaccharide to protein. The content of protein and polysaccharide in the conjugated vaccine wa...
Embodiment 3
[0104] Immune Effect Test of Bacterial Polysaccharides
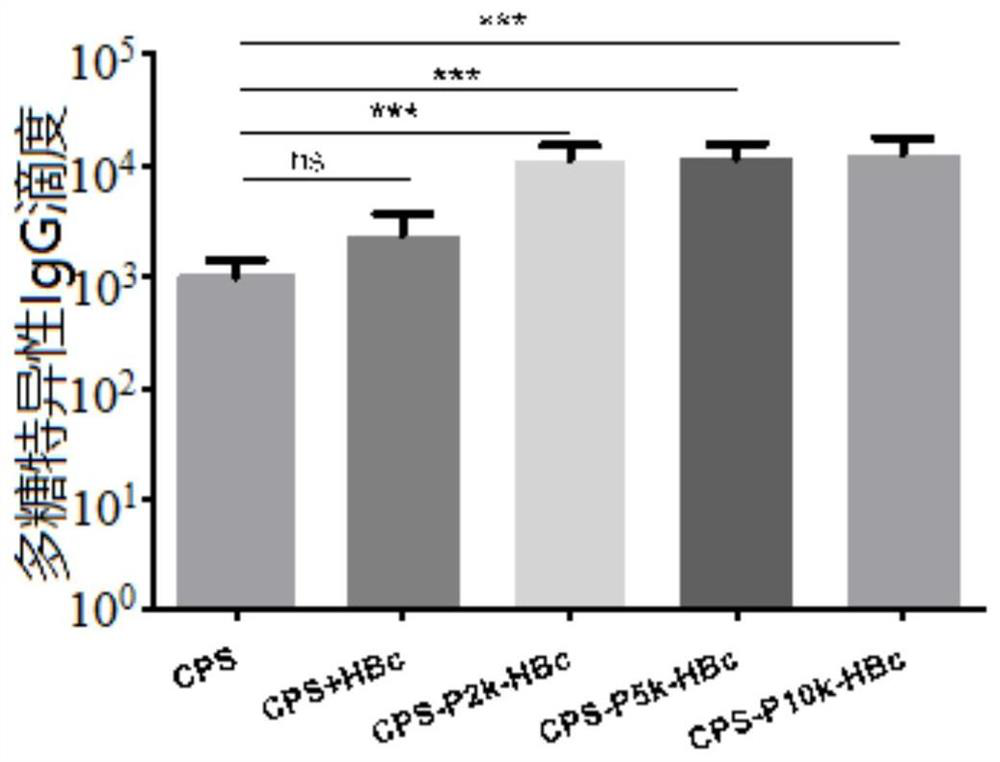
[0105] Select 36 BALB / c mice, 18-22 g, female, and randomly divide them into 5 groups, 6 mice in each group. A total of 6 groups of experiments were carried out: pure sugar group (CPS group), CPS and HBc physical mixture group (CPS+HBc group), CPS-P2k-HBc group, CPS-P5k-HBc group, CPS-P10k-HBc group. All groups were injected subcutaneously, and each animal was injected with a vaccine containing 2.5 μg of polysaccharide, once a week, for a total of 3 injections. One week after the third injection, blood was collected by tail docking.
[0106] (1) First, test the immunogenicity of different groups of vaccines
[0107] Anti-capsular polysaccharide IgG, IgG1 and IgG2a antibody titers and polysaccharide-specific IgG2a / IgG1 antibody titer ratios in mouse serum were detected by ELISA. Test results such as Figure 3A , Figure 3B , Figure 3C and Figure 3D As shown, the antibody titer of the polysaccharide conjugate vacc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com