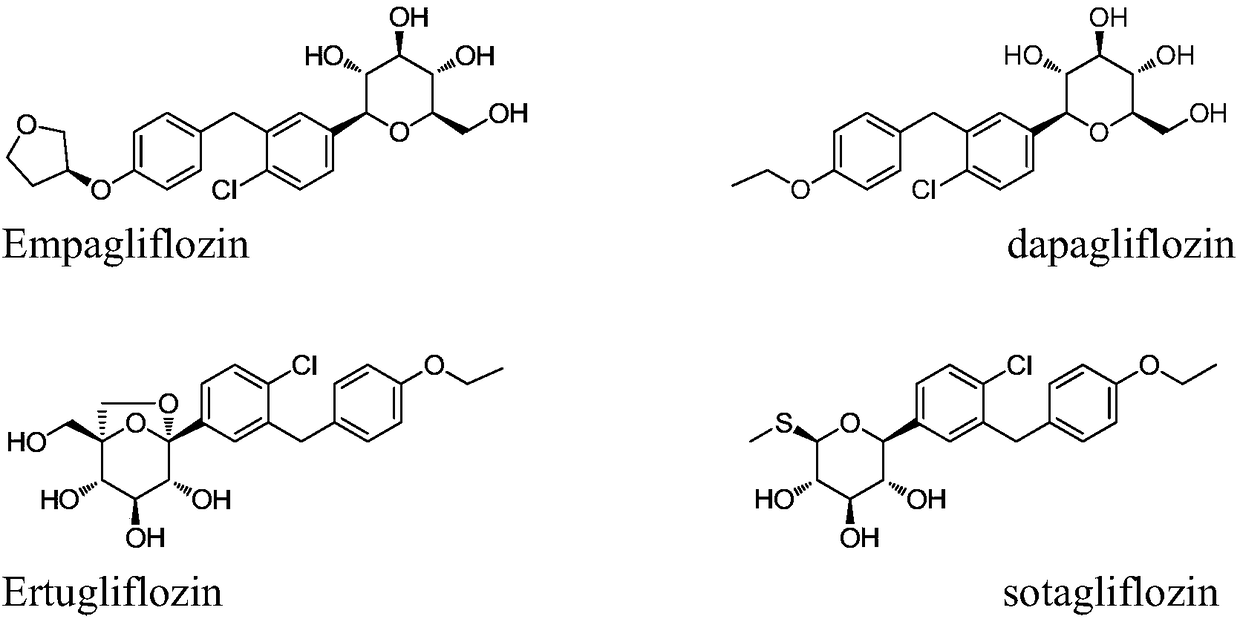
Method for preparing SGLT2 (Sodium-Dependent Glucose Transporters 2) inhibitor intermittent
A technology of intermediates and inhibitors, which is applied in the field of drug synthesis, can solve the problems of high COD, inability to recycle, and difficult storage of metal ethoxide reagents, and achieve the effects of reducing COD, easy recycling, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1 (5-iodo-2-chlorophenyl) (4-fluorophenyl) ketone synthesis:
[0040] Add (282g, 1mol) 5-iodo-2-chlorobenzoic acid, 1.0L dichloromethane, (142.8g, 1.2mol) thionyl chloride and a catalytic amount of DMF to the reaction flask, heat up to 39°C-42°C for 3hrs -5hrs. Cool down to 5-10°C, add (160g, 1.2mol) aluminum trichloride and (125g, 1.3mol) fluorobenzene, and heat to reflux overnight. After the reaction is complete, cool down to 0°C, add 100ml of 6N hydrochloric acid dropwise to quench the reaction, add 400ml of water to remove the organic phase, wash the organic phase with 400ml of saturated sodium bicarbonate solution, 400ml of saturated sodium chloride solution, dry over anhydrous sodium sulfate, and concentrate to Dry, add 100ml of absolute ethanol and heat to dissolve, cool down to 5-10°C, keep warm and crystallize for 2h, filter with suction, and dry to obtain white solid (5-iodo-2-chlorophenyl)(4-fluorophenyl)methanone 310g.
Embodiment 2
[0041] Embodiment 2 (5-iodo-2-chlorophenyl) (4-ethoxyphenyl) ketone:
[0042] Add (18g, 50mmol) (5-iodo-2-chlorophenyl) (4-fluorophenyl) methanone, 100ml absolute ethanol and (6.8g, 100mmol) sodium ethoxide to the reaction flask, and raise the temperature to 50°C-65 ℃ reaction, reacted for 1 hour, TLC monitored the reaction process, and there were obvious impurities; continued reaction for 3 hours until the raw materials basically disappeared, poured the reaction solution concentrated under reduced pressure to 20-30ml into ice water, precipitated solid, filtered, and washed the filter cake with water. After drying and passing through the column, 8.1 g of product compound I (5-iodo-2-chlorophenyl)(4-ethoxyphenyl)methanone was obtained, with a yield of 42%.
Embodiment 3
[0043] Embodiment 3 (5-iodo-2-chlorophenyl) (4-ethoxyphenyl) ketone:
[0044] Add (180g, 0.5mol) (5-iodo-2-chlorophenyl) (4-fluorophenyl)methanone, 900ml absolute ethanol and (40g, 1mol) NaOH to the reaction flask, and heat up to 50°C-65°C React for 5h-8h. After the reaction is over, distill 600ml-700ml of ethanol under reduced pressure, cool to 20°C-30°C, then pour the reaction solution into 300g-500g of ice water, filter, wash the filter cake with 300ml of water, and dry to obtain the product compound I(5-iodo-2-chlorophenyl)(4-ethoxyphenyl)methanone 182g, yield 94.3%, HPLC purity 99.5%.
[0045] The reaction result of embodiment 2 and 3 illustrates, when replacing sodium hydroxide with sodium ethylate, reaction yield also obviously reduces.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com