Preparation method of protoporphyrin disodium
A technology of disodium porphyrin and porphyrin diester, applied in directions such as organic chemistry, can solve the problems of difficult separation of products, low final yield, and many reaction steps, and achieve the effects of easy operation, improved purity, and improved yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The invention provides a kind of preparation method of disodium protoporphyrin, comprising the following steps:
[0030] (1) Protoporphyrin, anhydrous methanol and concentrated sulfuric acid undergo an alkyd reaction under ultrasonic conditions to obtain a protoporphyrin diester;
[0031] (2) Saponifying the protoporphyrin diester, NaOH methanol solution and toluene obtained in the step (1) under ultrasonic conditions to obtain disodium protoporphyrin.
[0032] In the invention, protoporphyrin, anhydrous methanol and concentrated sulfuric acid undergo alkyd reaction under ultrasonic conditions to obtain protoporphyrin diester. In the present invention, the ultrasonic frequency of the ultrasonic condition is preferably (0,80]KHz, more preferably [20,60]KHz. In the present invention, the power of the ultrasonic condition is preferably 0-400W, more preferably 100-400W.
[0033] In the present invention, the dosage ratio of the protoporphyrin, anhydrous methanol and conce...
Embodiment 1
[0064] Add hemin and acetic anhydride at a mass ratio of 1:45, measure acetic anhydride into an Erlenmeyer flask, then slowly add 1 mL of concentrated hydrochloric acid dropwise in a cold water bath, add 0.1 g of hemin and perform ultrasonic treatment, After reacting in a 40KHz, 250W ultrasonic reactor for 10 minutes, add 0.08 g of ferrous sulfate, and continue the ultrasonic reaction for 1 hour. After the reaction is over, add 4mol / L sodium hydroxide solution dropwise to adjust the pH of the solution to 5, take the precipitate after centrifugation, wash the precipitate twice with weakly acidic deionized water with a pH value of 4, and then continue to wash 3 times with deionized water . The crude product was purified by recrystallization with hot acetone and dried to obtain protoporphyrin.
[0065] Dissolve 0.1 g of protoporphyrin in 30 mL of anhydrous methanol, transfer them to a conical flask, add 0.7 mL of concentrated sulfuric acid, and sonicate at an ultrasonic frequenc...
Embodiment 2
[0069] Same as Example 1, the only difference is that the ultrasonic frequencies in the preparation process of protoporphyrin diester are 0, 20, 60, 80 KHz respectively.
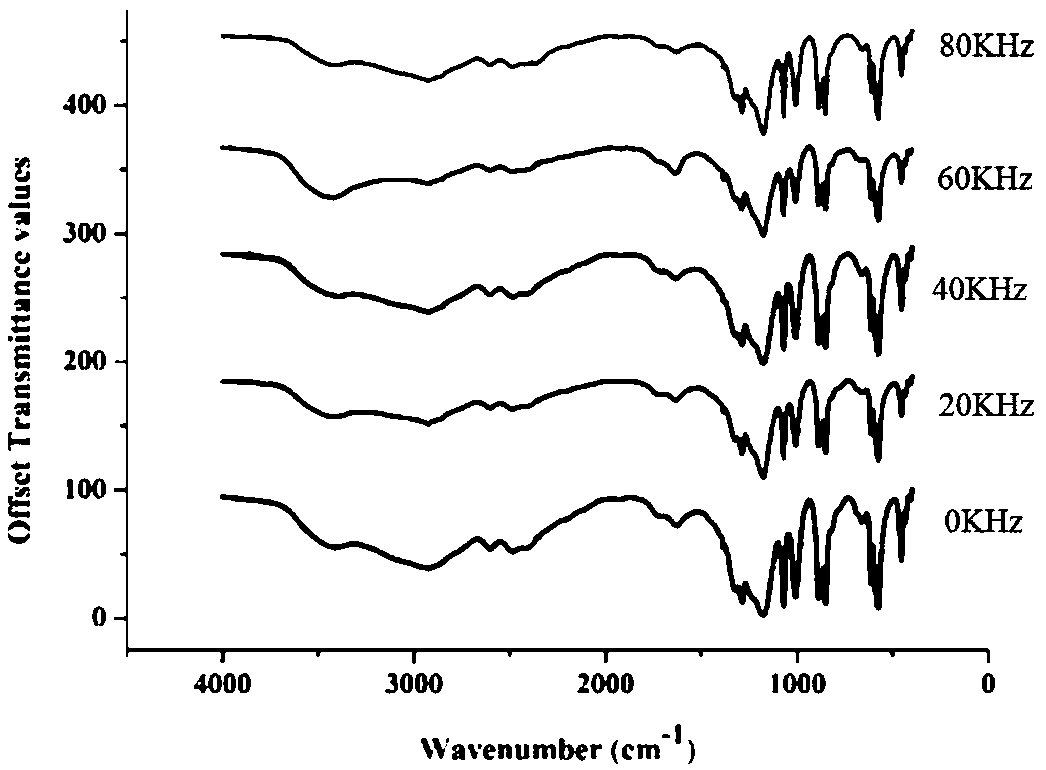
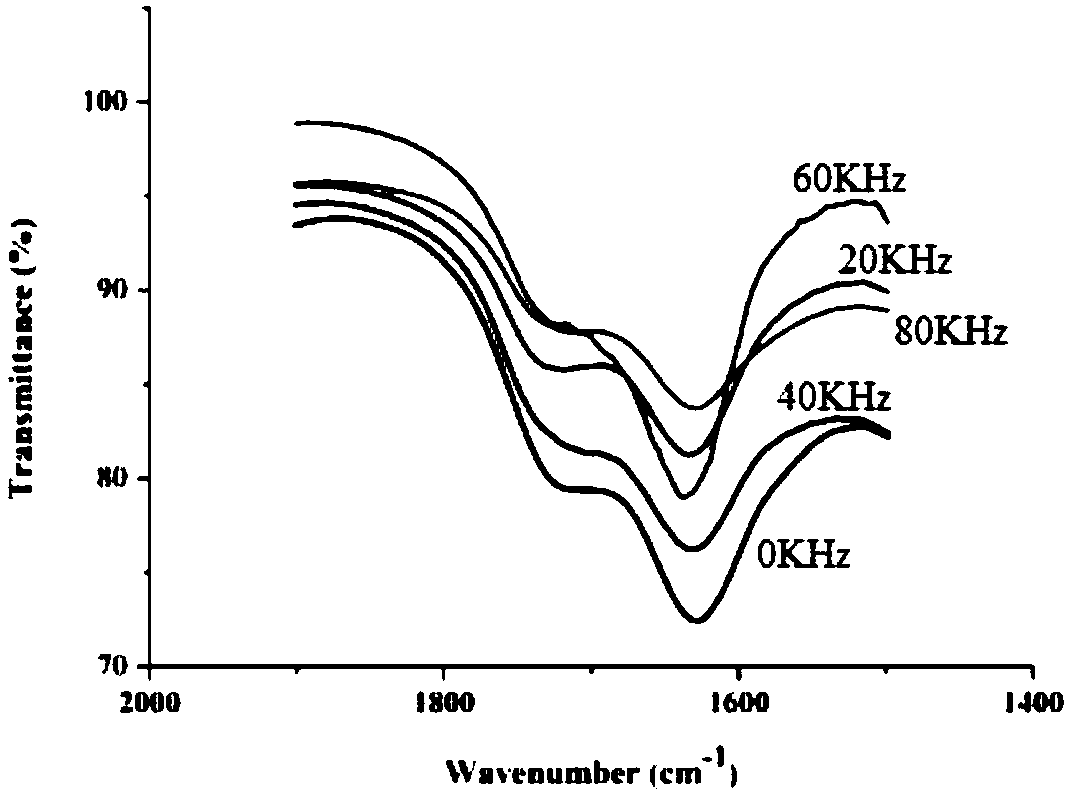
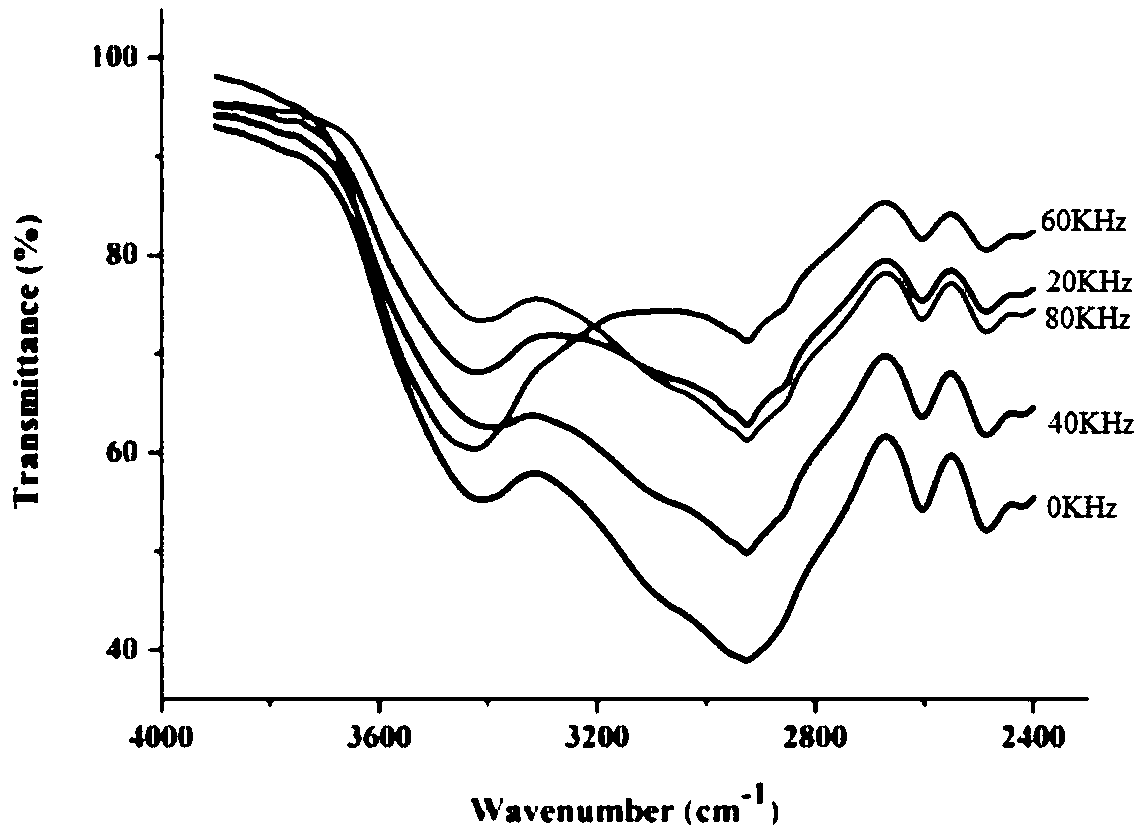
[0070] The protoporphyrin diester that embodiment 1~2 makes carries out infrared spectrum research, and the result is as follows Figure 1~3 as shown, figure 1 Infrared wavelength 4000 ~ 400cm -1 Infrared spectrum, figure 2 Infrared wavelength 2000~1400cm -1 Infrared spectrum, image 3 Infrared wavelength 4000~2400cm -1 infrared spectrum. Depend on Figure 1~3 It can be seen that the protoporphyrin diesters prepared by the present invention are at 1697~1722cm -1 Weak peaks appear in the range, and the absorption peak in this band corresponds to the characteristic peak of C=O in the ester group. Affected by factors such as the synthesis method of the sample and its own purity, the same functional group will shift. 1600~1650cm -1 The existence of the characteristic peak of C=C at 1 shows that during...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com