A preparation method of inorganic halogen perovskite fluorescent quantum dots through one-step synthesis and a product thereof
A technology of fluorescent quantum dots and perovskite, applied in chemical instruments and methods, nano optics, luminescent materials, etc., can solve the problems of increased cost of preparation requirements, reduced product quality and monodispersity, and reduced material synthesis efficiency, etc., to achieve The preparation method is simple and feasible, the reaction conditions are not harsh, and the effect of simple and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Weigh 0.0605g of PbCl 2 , 0.030g CsCO 3 (molar ratio 2:1), added to 5mL of ODE, and added 2mL oleic acid and 1mL oleylamine (volume ratio 5:2:1), heated and stirred at 170°C for 20min, cooled in an ice-water bath after the reaction After reaching room temperature, the solution obtained by the reaction was centrifuged, the supernatant was removed, centrifuged three times with n-hexane, and dried to obtain a solid powder. Such as figure 1 As shown, the inorganic halogen perovskite fluorescent quantum dots prepared in this embodiment have a maximum emission wavelength of 455 nm, a crystal structure of perovskite structure, and a fluorescence quantum yield of 40%.
Embodiment 2
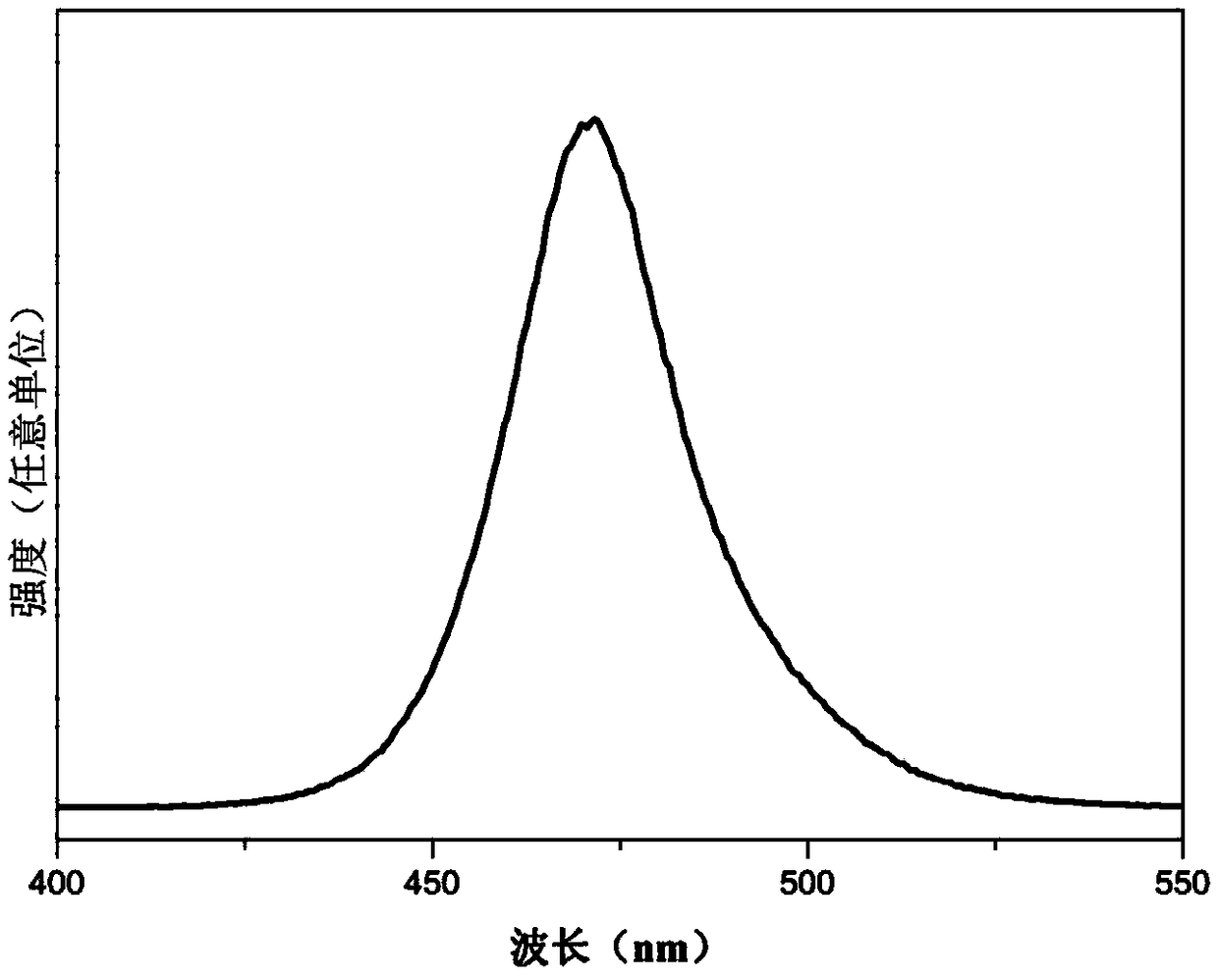
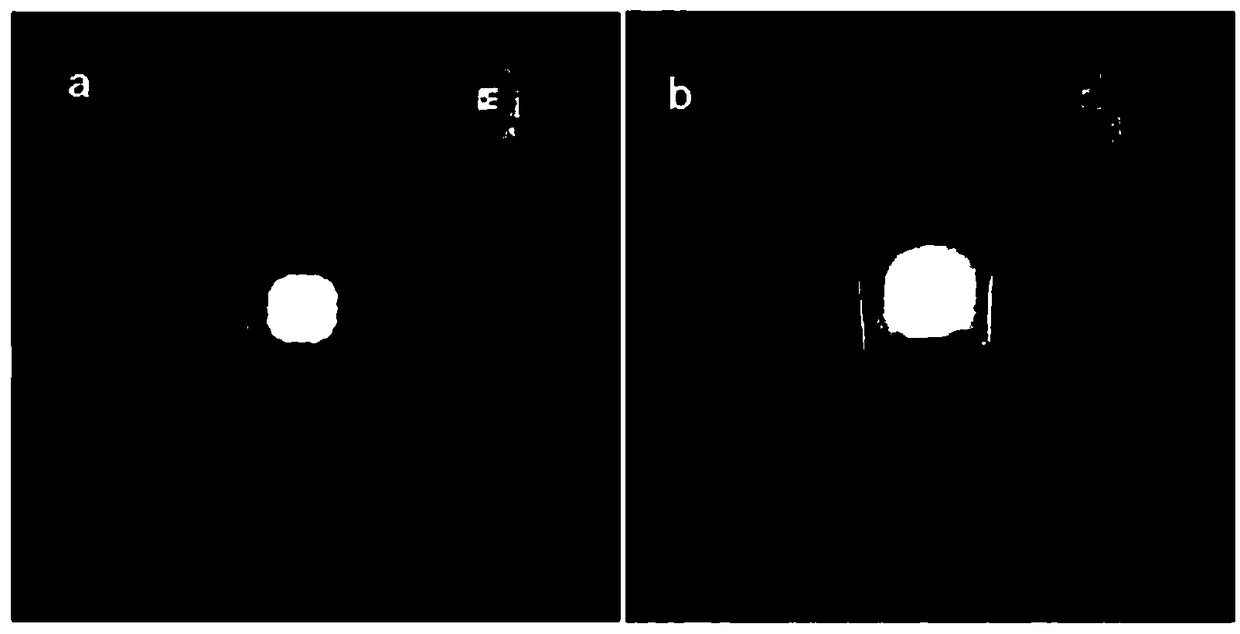
[0028] Weigh 0.68g PbBr 2 , 0.30g CsCO 3 (molar ratio 2:1), add to 50mL ODE, and add 20mL oleic acid and 10mL oleylamine (volume ratio 5:2:1), heat and stir at 120°C for 20min, cool in an ice-water bath after the reaction to room temperature; then the solution obtained by the reaction was centrifuged, the supernatant was removed, centrifuged 3 times with n-hexane, and dried to obtain a solid powder, about 11g, such as figure 2 As shown, wherein a is weighing under natural light, b is weighing under ultraviolet irradiation, and it can be seen that the products all emit fluorescence. All operations are carried out under normal pressure without protective gas. Such as image 3 As shown in (a), under the excitation of a blue LED, the inorganic halogen perovskite fluorescent quantum dots prepared in this example can emit bright green light (b) without blue light leakage. Figure 4 Spectrum of the inorganic halogen perovskite fluorescent quantum dots prepared for this example u...
Embodiment 3
[0030] Weigh 0.085g PbI 2 , 0.030g CsCO 3 (molar ratio 2:1), added to 5mL of ODE, and added 2mL oleic acid and 1mL oleylamine (volume ratio 5:2:1), heated and stirred under vacuum at 120°C for 20min, after the reaction was completed, placed in an ice-water bath Cool to room temperature; centrifuge the solution obtained from the reaction, remove the supernatant, centrifuge 3 times with n-hexane, and dry to obtain a solid powder. Such as Image 6 It is shown that the inorganic halogen perovskite fluorescent quantum dots prepared in this example have a maximum emission wavelength of 595nm, as shown in Figure 7 Its fluorescence lifetime is shown as 5.7 ns and its size is about 20 nm.
[0031] Verified by experiments, PbX 2 with CsCO 3 The molar ratio of 2-4:1 all can realize reaction, and wherein X is selected from Cl, Br, I, PbX 2 The volume ratio of the amount of substance to octadecene is calculated as 1mol:25-27L; the volume ratio of octadecene, oleic acid and oleylamin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com