Metal titanium preparation method combining hydrogen reduction of TiO2 and molten salt electrolysis of Ti4O7
A technology of molten salt electrolysis and metal titanium, applied in the field of metallurgy, can solve the problems of long process flow, low deoxidation efficiency, long electrolysis time, etc., and achieve the effects of low energy consumption, improved current efficiency, and short time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
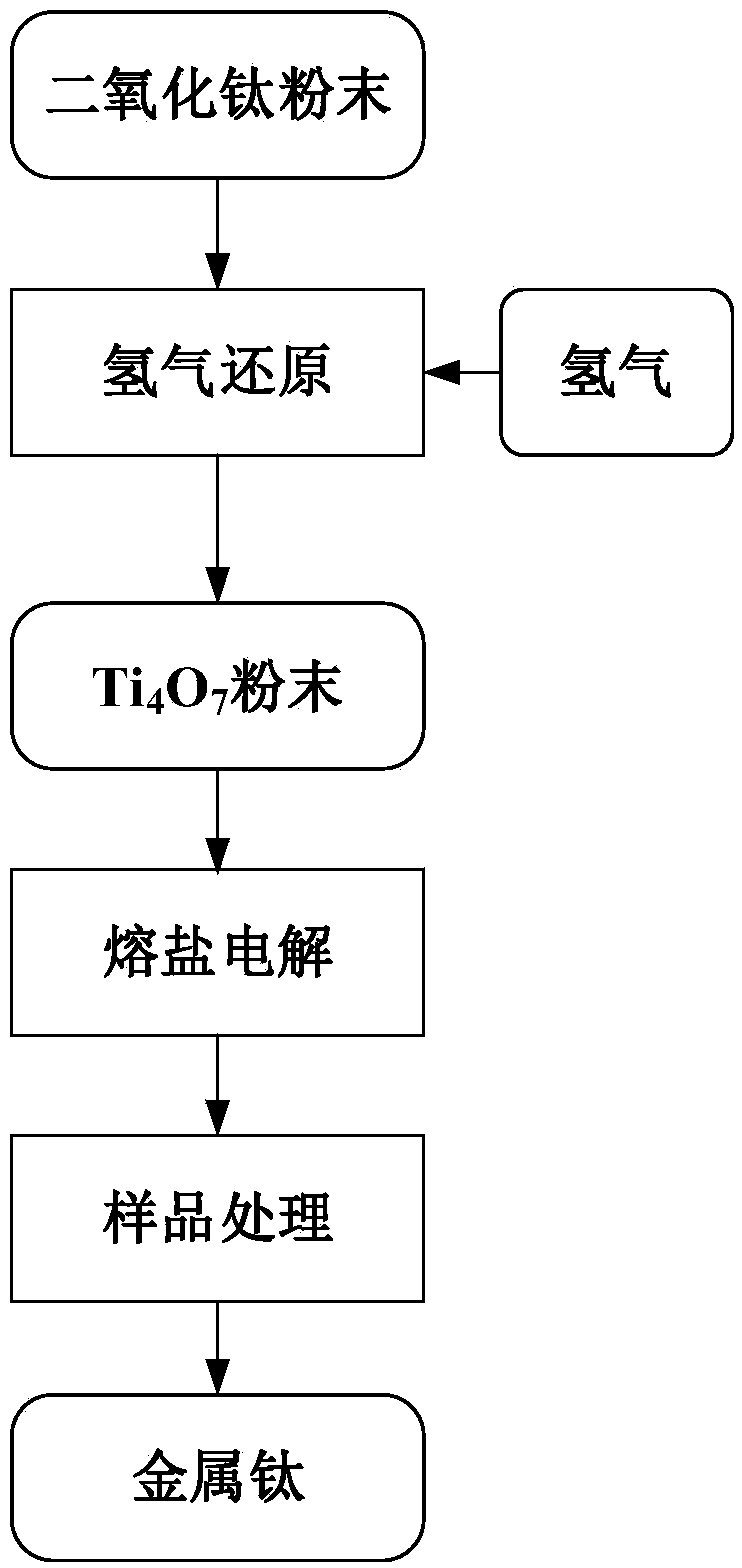
[0032] The technological process of this embodiment is as figure 1 shown. Choose TiO with a purity of ≥99.8% and pass through a 140-mesh sieve 2 powder, adopt the method of the present invention to prepare metal titanium, wherein the time of molten salt electrolysis is 1 hour, and the steps are as follows:
[0033] (1) TiO 2 The powder is spread on the corundum crucible and put into the sealed tubular heating furnace, and the thickness of the laying is controlled below 3mm. After closing the furnace cover, continue to feed argon (the flow rate of argon gas is 250ml / min) to ensure the inert atmosphere in the furnace. After 3 hours, the temperature in the furnace rises from room temperature to 1300°C.
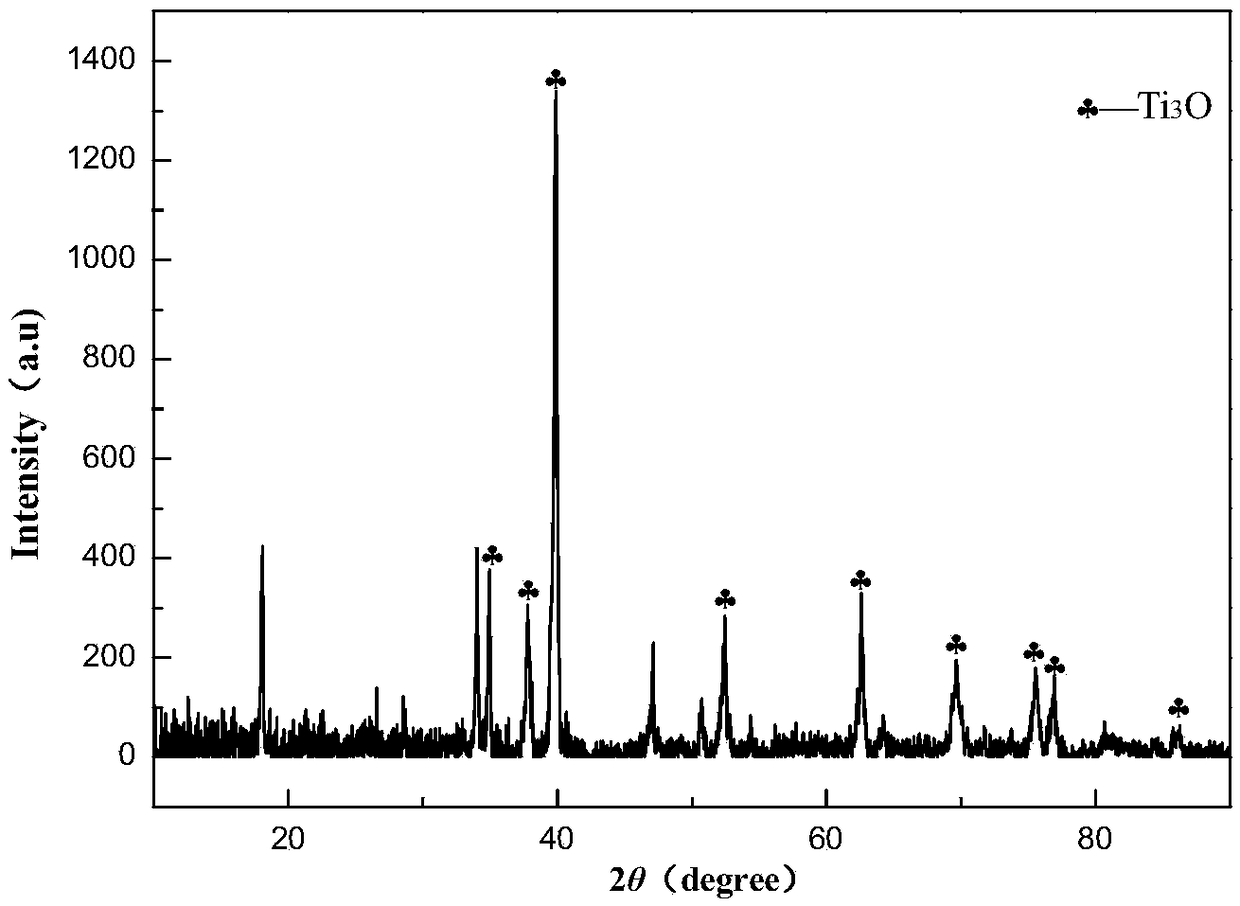
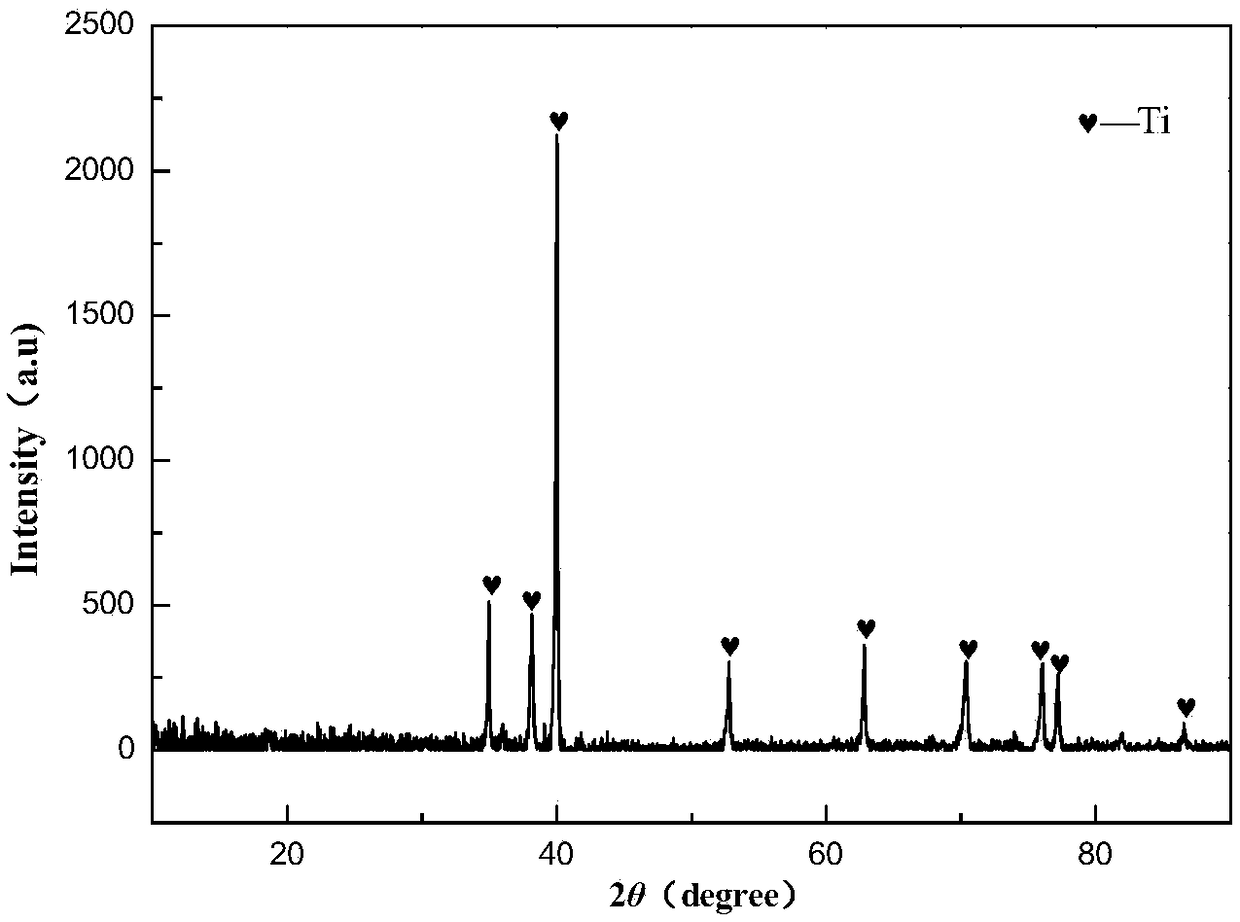
[0034] (2) After the temperature is stabilized, stop feeding argon gas and change it to hydrogen gas (hydrogen gas flow rate is 250ml / min), and keep the temperature at 1300°C for 2 hours, and the obtained Ti 4 o 7 Samples were wrapped with 400-mesh stainless steel gauze as t...
Embodiment 2
[0041] The present embodiment selects the TiO that purity is ≥ 99.8% and crosses 140 mesh sieves for use 2 powder, adopt the method of the present invention to prepare metal titanium, wherein the time of molten salt electrolysis is 6 hours, and the steps are as follows:
[0042] (1) TiO 2 The powder is spread on the corundum crucible and put into the sealed tubular heating furnace, and the thickness of the laying is controlled below 3mm. After closing the furnace cover, continue to feed argon (the flow rate of argon gas is 250ml / min) to ensure the inert atmosphere in the furnace. After 3 hours, the temperature in the furnace rises from room temperature to 1300°C.
[0043] (2) After the temperature is stabilized, stop feeding argon gas and change it to hydrogen gas (hydrogen gas flow rate is 250ml / min), and keep the temperature at 1300°C for 2 hours, and the obtained Ti 4 o 7 Samples were wrapped with 400-mesh stainless steel gauze as the cathode raw material for the electro...
Embodiment 3
[0050] The present embodiment selects the TiO that purity is ≥ 99.8% and crosses 140 mesh sieves for use 2 powder, adopt the method of the present invention to prepare metal titanium, wherein the time of molten salt electrolysis is 3 hours, and the steps are as follows:
[0051] (1) TiO 2 The powder is spread on the corundum crucible and put into the sealed tubular heating furnace, and the thickness of the laying is controlled below 3mm. After closing the furnace cover, continue to feed argon (the flow rate of argon gas is 250ml / min) to ensure the inert atmosphere in the furnace. After 3 hours, the temperature in the furnace rises from room temperature to 1300°C.
[0052] (2) After the temperature is stabilized, stop feeding argon gas and change it to hydrogen gas (hydrogen gas flow rate is 250ml / min), and keep the temperature at 1300°C for 2 hours, and the obtained Ti 4 o 7 Samples were wrapped with 400-mesh stainless steel gauze as the cathode raw material for the electro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com